Method for separation and determination of Palbociclib intermediate Z1 and related substances
A technology related to substances and intermediates, applied in the field of analytical chemistry, to achieve the effect of strong specificity and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Example 1 Method for separating and determining palbociclib intermediate Z1 and related substances by high performance liquid chromatography
[0100] (1) Prepare blank solution (diluent): take 25ml of 0.1% trifluoroacetic acid aqueous solution, put it in a 100ml measuring bottle, add acetonitrile to dilute to the mark, shake well, and you get it;
[0101] (2) Prepare impurity localization solution: take palbociclib intermediate Z1 impurity reference substance, dissolve and dilute with diluent to obtain impurity localization solution;
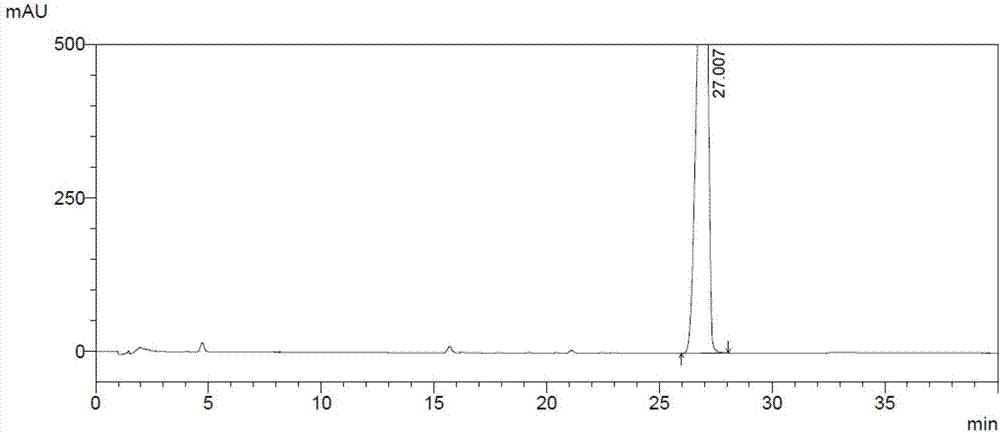
[0102] (3) Preparation of the test solution: get about 25 mg of palbociclib intermediate Z1, accurately weighed, put in a 50ml measuring bottle, add diluent to dissolve and dilute to the scale, shake up, as the test solution;
[0103] (4) Prepare self-contrast solution: accurately pipette 1.0ml of the test solution, put it in a 100ml measuring bottle, add diluent to dilute to the mark, shake well, and get final product;
[0104] (5) Prep...
Embodiment 2
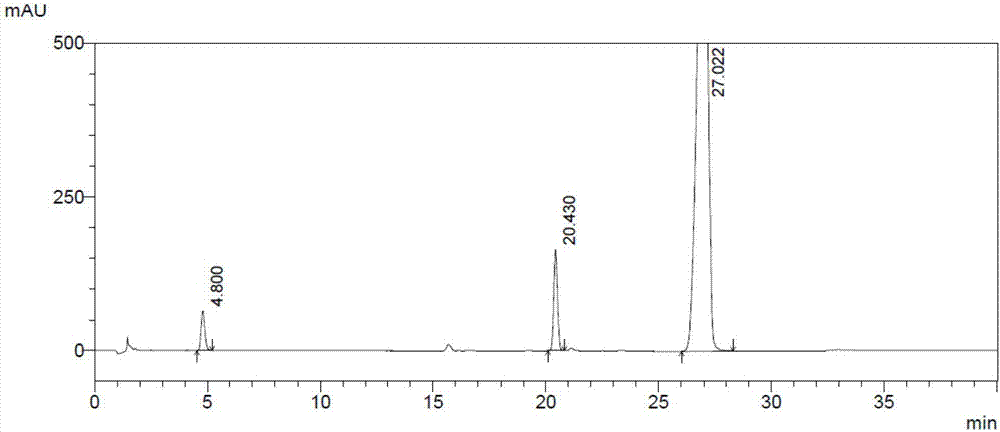
[0106] Example 2 The separation degree of palbociclib intermediate Z1 and its related substances by the high performance liquid chromatography system of the present invention
[0107] (1) Prepare blank solution (diluent): take 25ml of 0.1% trifluoroacetic acid aqueous solution, put it in a 100ml measuring bottle, add acetonitrile to dilute to the mark, shake well, and you get it;
[0108] (2) Preparation of the test solution: Accurately weigh 26.63mg of intermediate Z1, put it in a 50ml measuring bottle, add diluent to dissolve and dilute to the mark, shake well, and then get the test solution with a concentration of 0.5326mg / ml ;
[0109] (3) Preparation of impurity positioning solution:
[0110] Impurity 1 stock solution: Accurately weigh 13.83mg of impurity 1, put it in a 25ml measuring bottle, add diluent to dissolve and dilute to the mark, shake well, and the concentration is 553.2μg / ml;
[0111] Impurity 2 stock solution: Accurately weigh 11.32mg of impurity 2, put it ...
Embodiment 3
[0116] Example 3 The detection level of palbociclib intermediate Z1 and its related substances by the high performance liquid chromatography system of the present invention
[0117] (1) Prepare blank solution (diluent): take 25ml of 0.1% trifluoroacetic acid aqueous solution, put it in a 100ml measuring bottle, add acetonitrile to dilute to the mark, shake well, and you get it;
[0118] (2) Prepare the test solution: accurately weigh 26.63mg of intermediate Z1, put it in a 50ml measuring bottle, add diluent to dissolve and dilute to the mark, shake well, and the concentration is 0.5326mg / ml;
[0119] (3) Prepare self-contrast solution: accurately pipette 1.0ml of the test solution, put it in a 100ml measuring bottle, add diluent to dilute to the mark, shake well, and get final product;
[0120] (4) Prepare secondary dilution of intermediate Z1: accurately pipette 1.0ml of self-contrast solution, put it in a 10ml measuring bottle, add diluent to dilute to the mark, shake well, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| mobile phase | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com