Method for separation determination of posaconazole intermediate Z1 and related substances of posaconazole intermediate Z1
A related substance, posaconazole technology, applied in the field of analytical chemistry, to achieve the effect of strong specificity and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Embodiment 1 The method for the determination of related substances in posaconazole intermediate Z1 by high performance liquid chromatography
[0071] Specific method steps:
[0072] 1) Prepare a blank solution: take 40ml of water, put it in a 100ml measuring bottle, add acetonitrile to dilute to the mark, shake well, and you get it;
[0073] 2) Prepare related substance localization solution: take posaconazole intermediate Z1 related substance reference substance, dissolve and dilute with diluent to obtain impurity localization solution;
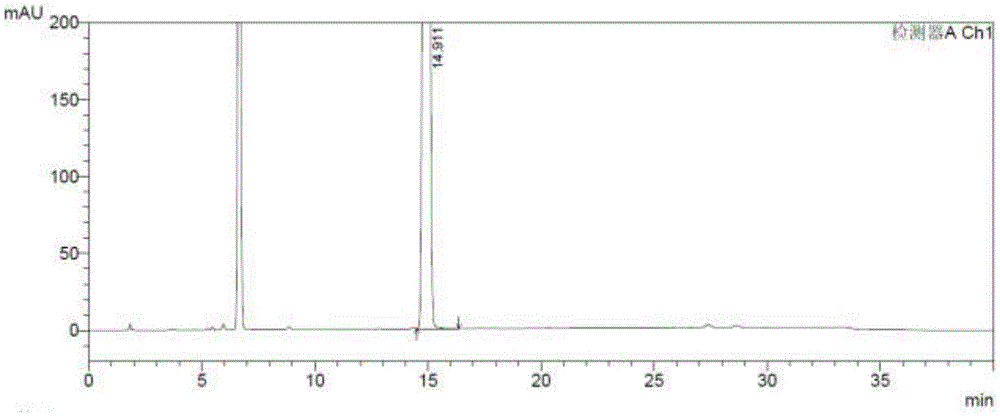
[0074] 3) Prepare the test solution: take about 50 mg of posaconazole intermediate Z1, accurately weigh it, put it in a 25ml measuring bottle, add diluent to dissolve and dilute to the mark, shake well, and use it as the test solution;
[0075] 4) Prepare self-contrast solution: accurately pipette 1.0ml of the test solution, put it in a 100ml measuring bottle, add diluent to dilute to the mark, shake well, then precisely pipette 1.0...
Embodiment 2
[0077] Example 2 The separation degree of the chromatographic system of the present invention to posaconazole intermediate Z1 and its related substances
[0078] Specific method steps:
[0079] 1) Prepare a blank solution: take 40ml of water, put it in a 100ml measuring bottle, add acetonitrile to dilute to the mark, shake well, and you get it;
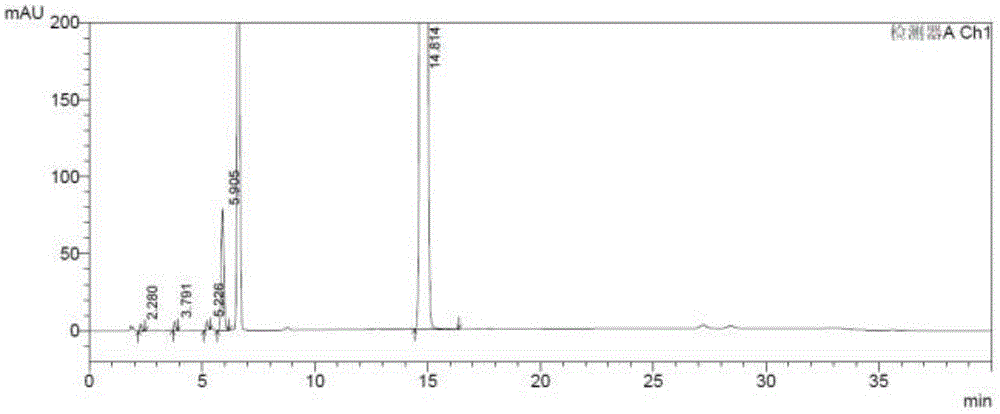
[0080] 2) Prepare the test solution: accurately weigh 149.85 mg of intermediate Z, put it in a 25 ml measuring bottle, add diluent to dissolve and dilute to the mark, shake well, and obtain; (1.994 mg / ml);
[0081] 3) Preparation of related substance positioning solution:
[0082] a) Impurity 1 stock solution: Accurately weigh 112.71mg of impurity, put it in a 25ml measuring bottle, add diluent to dissolve and dilute to the mark, shake well, and obtain; (508.4μg / ml);
[0083] b) Impurity 2 stock solution: Accurately weigh 212.70mg of impurity, put it in a 25ml measuring bottle, add diluent to dissolve and dilute to the mark, shake w...
Embodiment 3
[0090] Example 3 The system suitability test of the chromatographic system of the present invention to the posaconazole intermediate Z1
[0091] experiment procedure:
[0092] 1) Prepare a blank solution: take 40ml of water, put it in a 100ml measuring bottle, add acetonitrile to dilute to the mark, shake well, and you get it;
[0093] 2) Preparation of the test solution: take posaconazole intermediate Z149.85mg, accurately weighed, put in a 25ml measuring bottle, add diluent to dissolve and dilute to the scale, shake well, and obtain;
[0094] 3) Prepare self-contrast solution: accurately pipette 1.0ml of the test solution, put it in a 100ml measuring bottle, add diluent to dilute to the mark, shake well, then precisely pipette 1.0ml, put it in a 10ml measuring bottle, add diluent to dilute to the scale, shake well, and you get it; (0.1%);
[0095] 4) Take the self-control solution and inject 6 needles continuously, record the chromatogram, see Figure 4 Calculate the peak...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com