Method for separating and determining related substances in ezetimibe tablet
A technology for related substances and ezetimibe, which is applied in the field of separation and determination of related substances in ezetimibe tablets, achieving the effects of good specificity, broad application prospects, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
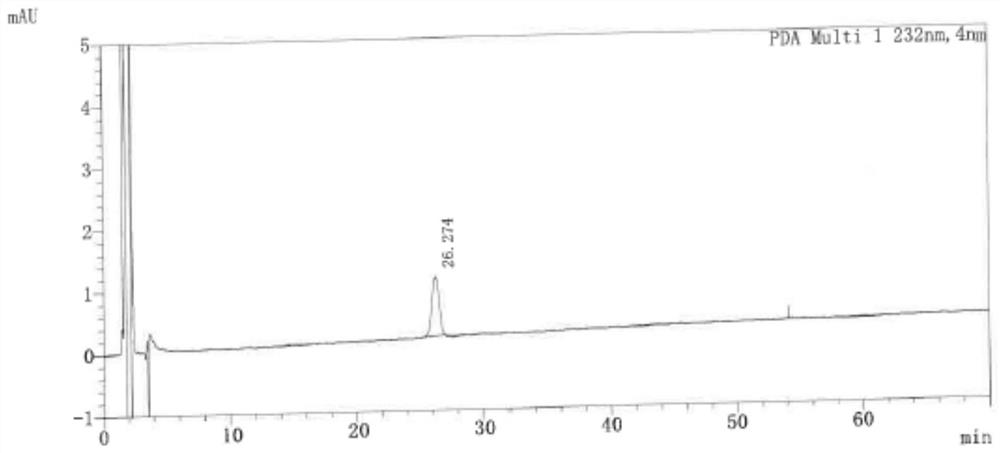
[0066] 1, instruments and conditions
[0067] Instrument: Liquid Chromatograph
[0068] Detector: UV detector
[0069] Columns: octadecyl silicone bile silica gel as filler (Hypersil BDS 150 × 4.6mm, 5 μm or) column)
[0070] Mobile phase (V: V): 0.05 mol / L of pelicate solution, acetonitrile, tetrahydrofuran is 65:25:10
[0071] Diluent (V: V): The volume ratio of glacial acetic acid, acetonitrile and water is 0.1: 60: 40
[0072] Test wavelength: 232nm
[0073] Column temperature: 25 ° C
[0074] Flow speed: 1.0ml / min
[0075] Inject volume: 30 μL.
[0076] 2, experimental steps
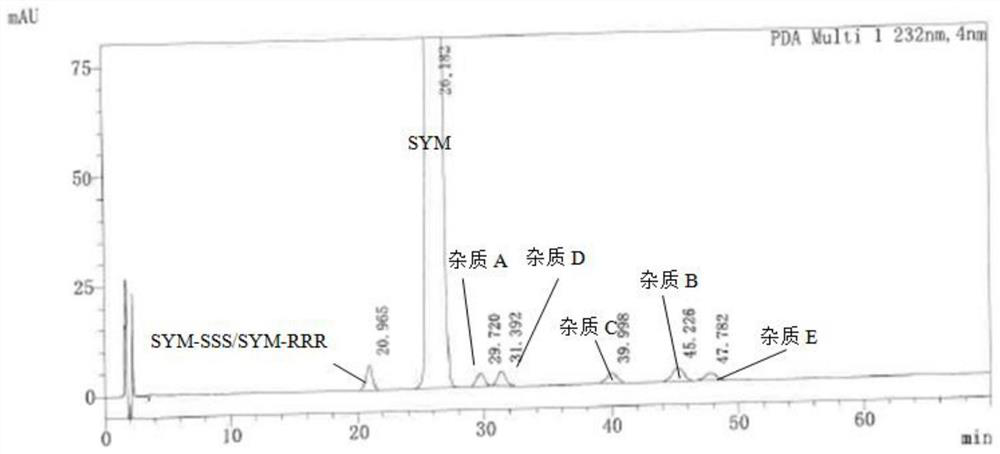
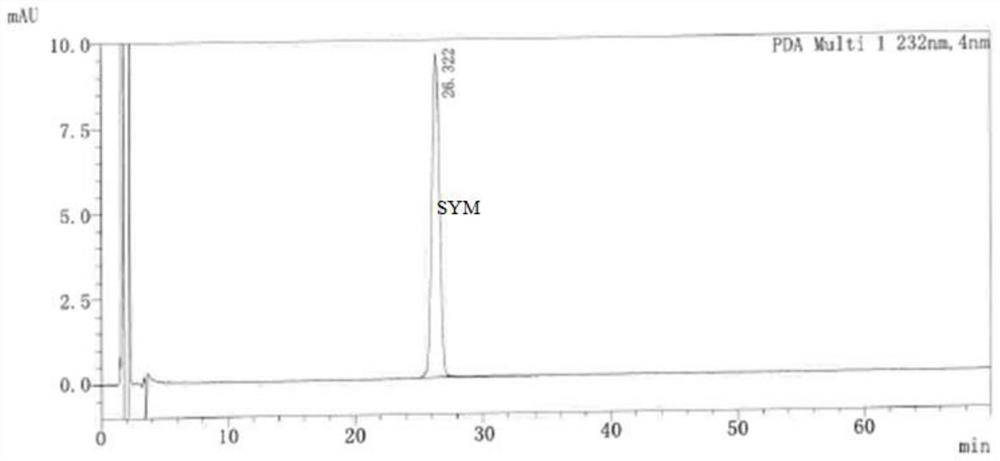
[0077] 2.1, use diluent to dissolve the containing folded wheat (concentration 1 mg / ml), related substance A, related substance B, related substance C, related substance D, related substance E, SYM-SSS / SYM-RRR 2 μg / ml Mixed solution, as a systematic solution;
[0078] 2.2, retrieving the right to fold the wheat powder is moderately, dissolved with a diluent, formulated into a solution of 10 mg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com