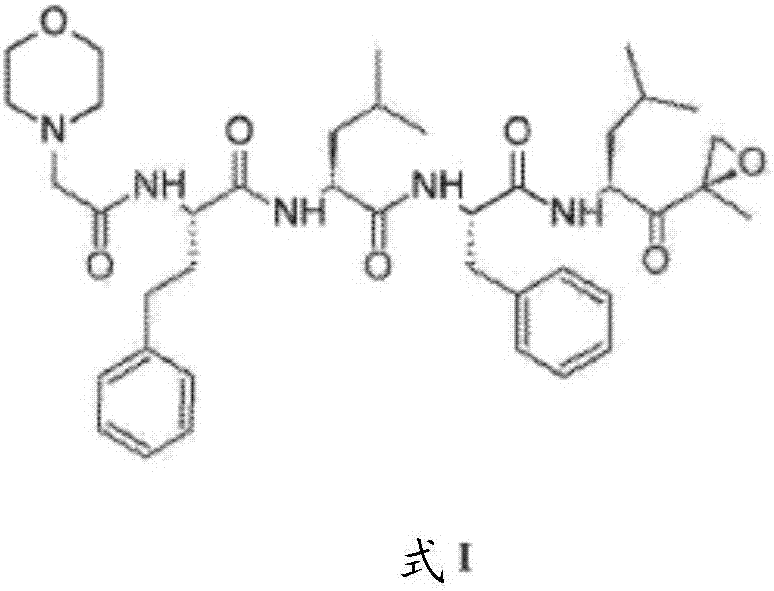
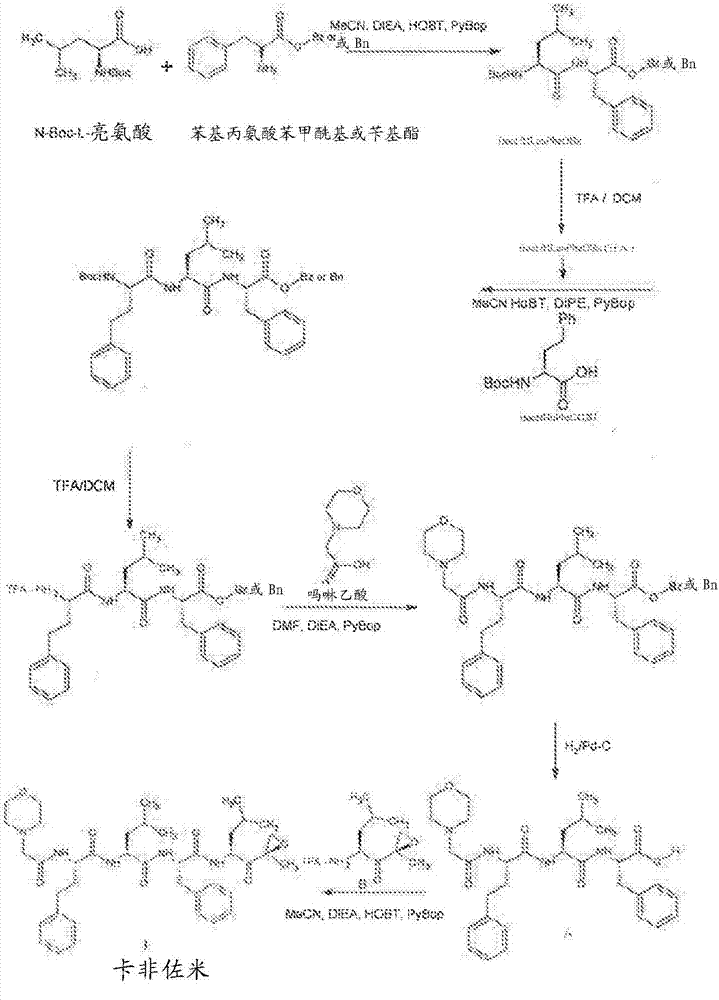
A process for purification of carfilzomib
一种卡非佐米、草酸卡的技术,应用在还原通常在合成卡非佐米期间形成的乙酰胺杂质领域,达到容易成本的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0063] a) preparation of carfilzomib oxalate:
[0064] Crude carfilzomib (10 g) and oxalic acid (1.31 g) were dissolved in a mixture of tetrahydrofuran (70 ml) and acetonitrile (50 ml), and stirred at 20-25°C for 1 hour.
[0065] After 1 hour the reaction mixture was cooled to 0-10°C and stirred for 3 hours, then the formed precipitate was filtered to give carfilzomib oxalate.
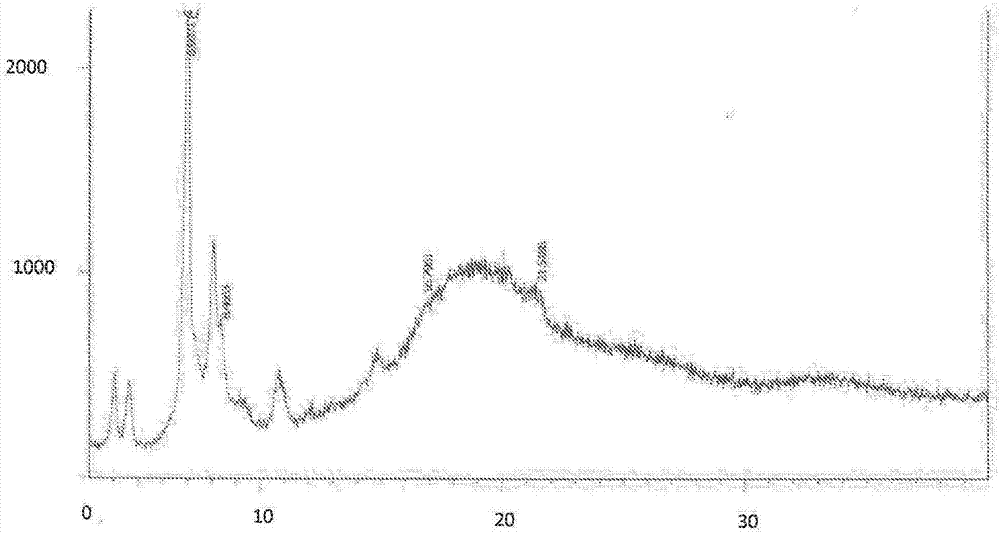
[0066] Carfilzomib oxalate: X-ray powder diffraction pattern (XRPD) with characteristic peaks at the following (2θ±0.2 degrees): 4.06, 4.66, 6.99, 8.07, 8.46, 9.30, 10.72 and 14.84
[0067] b) Preparation of Carfilzomib:
[0068] Precipitated carfilzomib oxalate as a wet cake obtained in Example-1 was dissolved in dichloromethane (100 ml), followed by washing with sodium bicarbonate solution (100 ml) and water (100 ml). The organic layer was concentrated under reduced pressure and the product was crystallized in ethyl acetate / methyl tert-butyl ether mixture (200ml) (3:7) to give carfilzomib (8g).
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com