Novel melatonin compounds and preparation methods thereof and applications in medicine
A technology for melatonin and compounds, applied to novel melatonin compounds and their preparation and medical applications, can solve the problems of tolerance, unfavorable medication, and unsuitability for use by most patients, and achieves The effect of improving oral utilization and prolonging half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
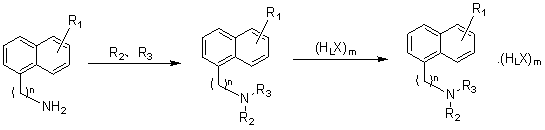
[0030] Embodiment 1: Preparation of N-((2-(3-methoxypropoxy)naphthalene-8-yl)ethyl)acetamide (compound I-1)
[0031]
[0032] Compound I-1
[0033] Take 10.0 g of (2-(3-methoxypropoxy)naphthalene-8-yl)methylamine and dissolve it in 100 ml of acetonitrile. Then 6.48 g of acetic anhydride was added. Heat to reflux for 6 hours. Add 1% activated carbon in total volume to the reaction liquid, reflux for decolorization for 15 min, and filter. The filtrate was concentrated to dryness under reduced pressure, and the residue was separated by HPLC to obtain 5.6 g of compound I-1.
Embodiment 2
[0034] Example 2: Preparation of N-((2-(3-(trifluoromethyl)propoxy)naphthalene-8-yl)ethyl)acetamide (Compound I-2)
[0035]
[0036] Compound I-2
[0037] Take 10.0 g of (2-(3-(trifluoromethyl)propoxy)naphthalene-8-yl)methylamine and dissolve it in 100 ml of acetonitrile. Then 6.48 g of acetic anhydride was added. Heat to reflux for 6 hours. Add 1% activated carbon in total volume to the reaction liquid, reflux for decolorization for 15 min, and filter. The filtrate was concentrated to dryness under reduced pressure, and the residue was separated by HPLC to obtain 5.0 g of compound I-2.
Embodiment 3
[0038] Embodiment 3: Preparation of (2-(3-methoxypropoxy)naphthalene-8-yl)-N,N-dimethylethylamine (compound 1-3)
[0039]
[0040] Compound I-3
[0041] Take 10.0 g of (2-(3-methoxypropoxy)naphthalene-8-yl)methanamine, add 100 ml of acetonitrile and stir to dissolve. Then 5.7 g of dimethyl sulfate was added. Heat to reflux for 6 hours. Add 1% activated carbon in total volume to the reaction liquid, reflux for decolorization for 15 min, and filter. The filtrate was concentrated to dryness under reduced pressure, and the residue was separated by HPLC to obtain 4.5 g of compound I-3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com