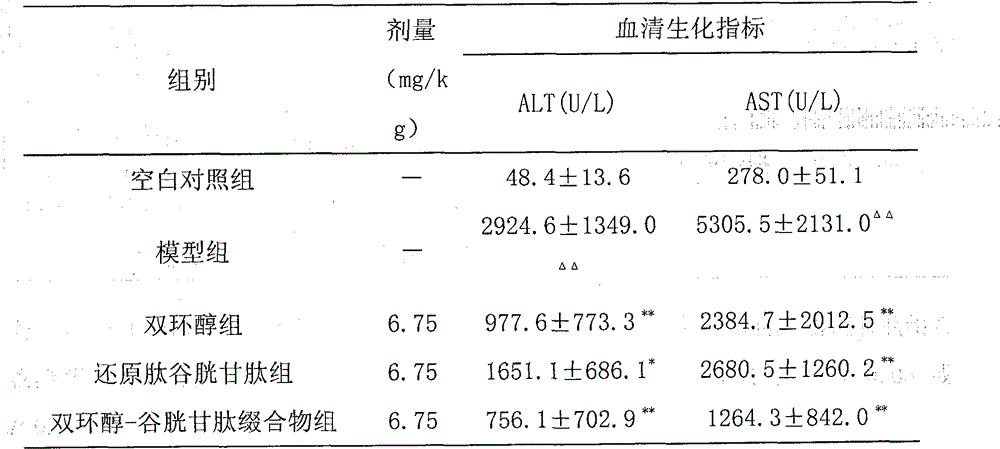
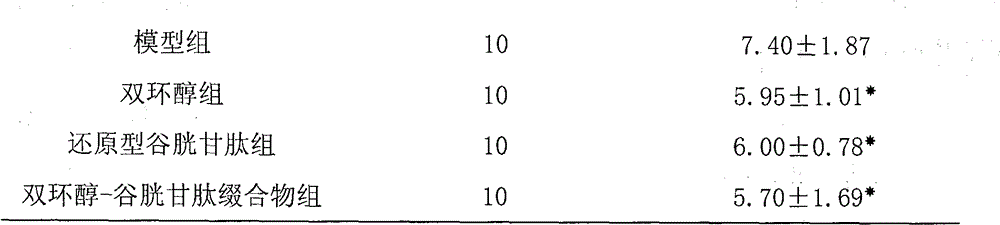
Bicyclol-glutathione conjugate and preparation method and application thereof
A glutathione and bicyclol technology, which is applied in the production of peptides and bulk chemicals, can solve the problems of low oral utilization, poor water solubility of bicyclol, and weak antiviral ability, achieving good water solubility and low cost. Effects of low and significant antiviral capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Preparation of Bicyclic Alcohol-Glutathione Conjugate.
[0047] (1) Protection of glutathione sulfhydryl group
[0048] Glutathione (5g, 16.27mmol) was added to 50mL DMF, then Mmt-Cl (5.6g, 18.1mmol) was added, and the reaction was stirred at room temperature for 12h. The reaction solution was evaporated to dryness, recrystallized with dichloromethane and petroleum ether, and filtered to obtain 7.1 g of white solid, that is, compound 12. [M-H] - =578.1.
[0049]
[0050] (2) Co-protection of sulfhydryl-protected glutathione α-position carboxyl and amino groups
[0051] Compound 12 (5.95g, 10mmol) was dissolved in 50mL THF, under nitrogen protection, 20mL of 1mol / L Et was added 3 B (20mmol) solution, reacted for 15h. The reaction solution was evaporated to dryness, recrystallized with acetonitrile and dichloromethane, and filtered to obtain (6.50) g of a white solid, namely compound 13.
[0052]
[0053] (3) Esterification reaction
[0054] Weigh...
Embodiment 2
[0060] Example 2 Preparation of Bicyclic Alcohol-Glutathione Conjugate.
[0061] (1) Protection of glutathione sulfhydryl group
[0062] Take 10mmol glutathione and add it to 50mL DMF, then add 12mmol Mob-Cl, and stir at 5°C for 24h. The reaction solution was evaporated to dryness, recrystallized with dichloromethane and petroleum ether, and filtered to obtain a white solid, thiol-protected glutathione.
[0063] (2) Co-protection of glutathione α-position carboxyl and amino groups
[0064] Take 5mmol of thiol-protected glutathione and dissolve it in 50mL THF, under nitrogen protection, add 5mmol of Et 3 B, react at 5°C for 20h. The reaction solution was evaporated to dryness, recrystallized with acetonitrile and dichloromethane, and filtered to obtain a white solid, which was protected glutathione.
[0065] (3) Esterification reaction
[0066] Weigh 3 mmol of protected glutathione and dissolve it in 20 mL of DCM, cool in an ice bath, add 40 mg of 4-dimethylaminopyridine...
Embodiment 3
[0069] Example 3 Preparation of Bicyclic Alcohol-Glutathione Conjugate.
[0070] (1) Protection of glutathione sulfhydryl group
[0071] Take 10mmol glutathione and add it to 50mL DMF, then add 12mmol Tmb-Cl, and stir at 40°C for 2h. The reaction solution was evaporated to dryness, recrystallized with dichloromethane and petroleum ether, and filtered to obtain a white solid, which was sulfhydryl-protected glutathione.
[0072] (2) Co-protection of glutathione α-position carboxyl and amino groups
[0073] Dissolve 5 mmol of sulfhydryl-protected glutathione in 50 mL of THF under nitrogen protection, add 7.5 mmol of 9-boronbicyclo[3.3.1]nonane dimer (25 mmol) solution, and react at 40°C for 10 h. The reaction solution was evaporated to dryness, recrystallized with acetonitrile and dichloromethane, and filtered to obtain a white solid, which was protected glutathione.
[0074] (3) Esterification reaction
[0075] Weigh 3 mmol of protected glutathione and dissolve it in 20 mL o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com