A kind of medicine for preventing or treating fxr-mediated disease and its preparation method and application
A drug and disease technology, applied in the field of drugs for preventing or treating FXR-mediated diseases and its preparation and use, can solve the problems of low oral utilization and short half-life, and achieve improved oral utilization and half-life prolonged effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
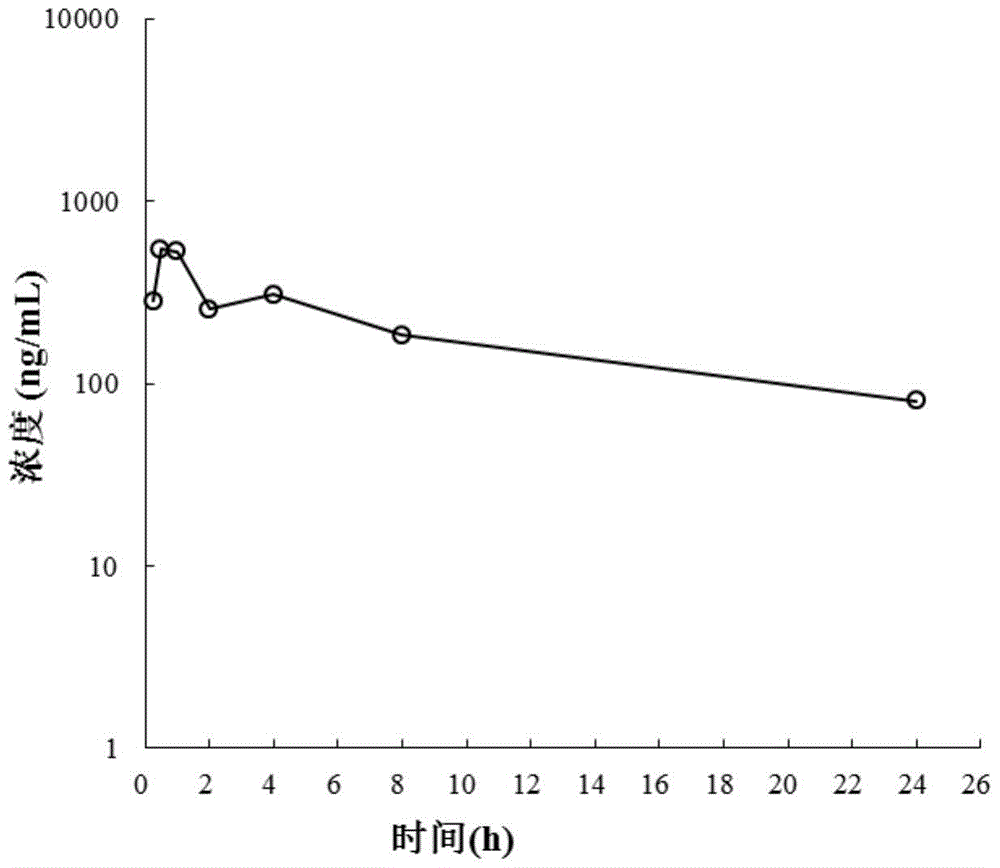
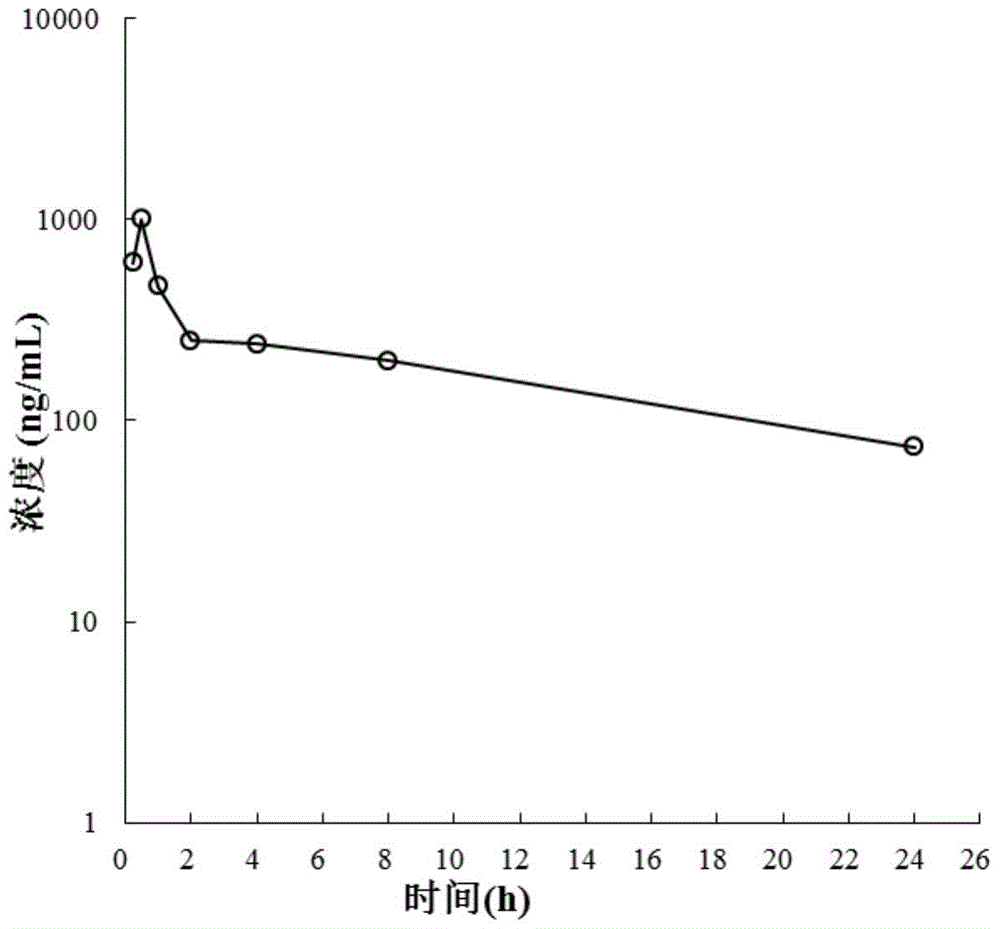
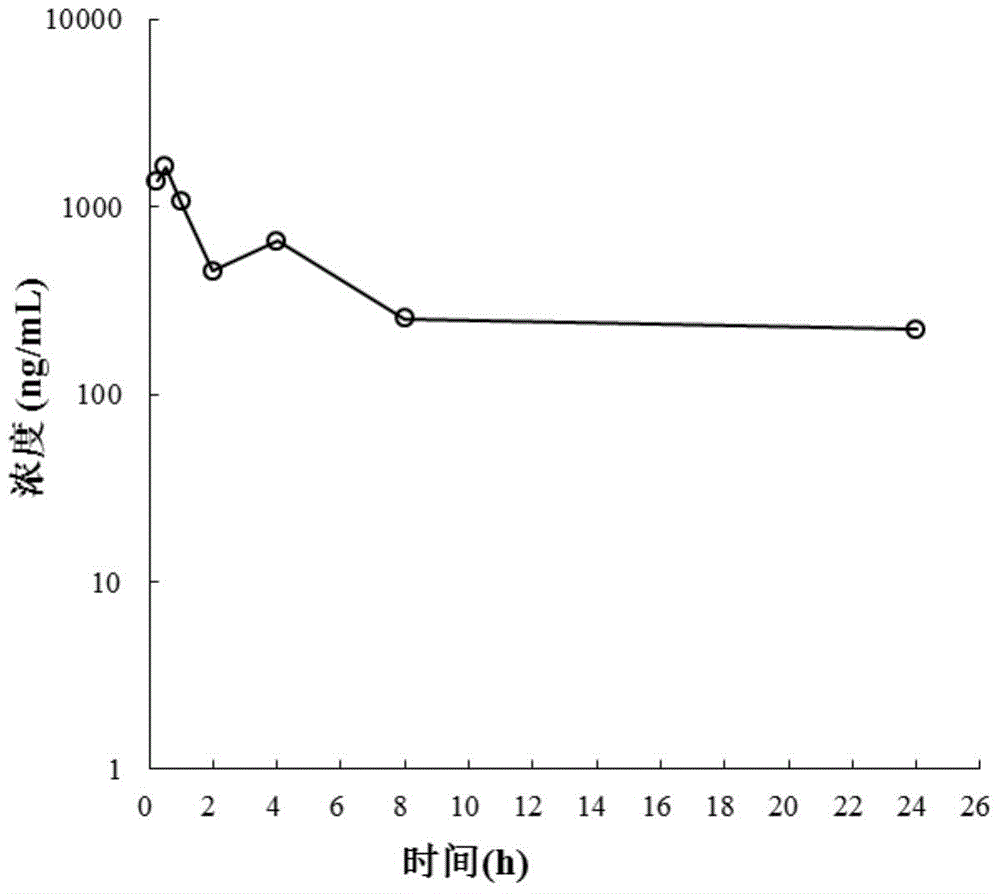
Image
Examples
Embodiment 1
[0042] The preparation of the compound shown in embodiment 1 formula (III)
[0043]
[0044] To a solution of compound 1 (2.5 g, 6.4 mmol) in methanol (60 mL) was added p-TsOH.H 2 O(p-toluenesulfonic acid monohydrate) (0.12 g, 0.64 mmol). The resulting mixture was stirred at room temperature for 3 hours. The solvent was evaporated under reduced pressure, and the residue was dissolved in EtOAc (ethyl acetate) (150 mL). NaHCO 3 (saturated, 100mL), water (50mL) and brine (50mL) washed twice, the organic layer was washed with Na 2 SO 4 Dried and evaporated under reduced pressure to obtain product 2 as a white solid (2.5 g, 96%).
[0045] 1 H NMR (400MHz, CDCl 3):δ3.66(s,3H),3.60(m,1H),2.85(dd,J=12.4,6.0Hz,1H),2.47-2.30(m,2H),2.28-2.20(m,2H), 2.05-1.60(m,10H),1.50-1.09(m,14H),0.92(d,3H),0.65(m,J=6.4Hz,3H).
[0046] To a solution of diisopropylamine (5.2 mL, 39.5 mmol) in dry tetrahydrofuran (30 mL) was added dropwise a solution of n-butyllithium (15.8 mL, 2.5M in n-he...
Embodiment 2
[0049] The preparation of embodiment 2 compound A
[0050]
[0051] To a solution of compound (III) (83 mg, 0.2 mmol) in MeOH (10 mL) was added TsOH (p-toluenesulfonic acid) (5.1 mg, 0.03 mmol). The mixture was stirred at room temperature for 16 hours. The solvent was evaporated under reduced pressure and the residue was dissolved in EtOAc (20ml), washed with saturated aqueous sodium bicarbonate (2x10ml), water (10ml) and brine (10ml). The organic layer was dried over anhydrous sodium sulfate and evaporated to dryness. The residue was purified by column chromatography on silica gel (eluted with DCM / MeOH (dichloromethane / methanol)=100:1 to 80:1) to obtain compound A as a white solid (42 mg, 50%).
[0052] 1 H NMR (400MHz, CDCl 3 ):3.70(s,1H),3.66(s,3H),3.43-3.37(m,1H),2.39-2.31(m,1H),2.26-2.18(m,1H),1.00(td,J=14.0 ,3.2Hz,1H),0.93-0.388(m,9H),0.66(s,3H).LC-MS: m / z 869.7[2M+H] + ,452.3[M+H 2 O] + .
Embodiment 3
[0053] The preparation of embodiment 3 compound B
[0054]
[0055] To a solution of compound (III) (83 mg, 0.2 mmol) in EtOH (10 mL) was added TsOH (5.1 mg, 0.03 mmol). The mixture was stirred at room temperature for 16 hours. The solvent was evaporated under reduced pressure, the residue was dissolved in EtOAc (20ml), washed with saturated aqueous sodium bicarbonate (2 x 10ml), water (10ml) and brine (10ml). The organic layer was dried over anhydrous sodium sulfate and evaporated to dryness. The residue was purified by column chromatography on silica gel (eluted with DCM / MeOH=100:1 to 80:1) to obtain compound B as a white solid (30 mg, 36%).
[0056] 1 H NMR (400MHz, CDCl 3 ):4.12(q,J=7.2Hz,2H),3.70(s,1H),3.43-3.35(m,1H),2.38-2.30(m,1H),2.26-2.18(m,1H),0.95- 0.86(m,12H),0.66(s,3H).LC-MS: m / z 897.7[2M+H] + ,466.4[M+H 2 O] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com