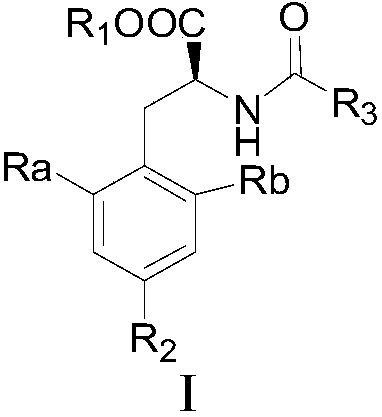
2',6'-dimethyltyrosine derivative and C-H activation methylation synthesis method thereof
A compound, alkoxymethyl technology, applied in the field of designing drug synthesis, can solve the problems of low efficiency, lengthy steps, harsh hydrogenation conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0222] Embodiment 1: Synthesis of methylation raw material 2a (using synthetic method one)
[0223]
[0224] Dissolve L-tyrosine (50mmol) in 50mL of dry methanol, cool the reaction to 0°C, then add SOCl dropwise 2 (75mmol). After the addition was complete, the reaction mixture was refluxed for 8 hours. The reaction was stopped, the reaction was cooled to room temperature, and the excess solvent was spun off to obtain the crude compound 4a as a white solid. The crude product of the compound can be directly subjected to the next reaction without separation by column chromatography.
[0225] Compound 4a (50 mmol) was dissolved in 70 mL of dry acetonitrile, and the reaction solution was cooled to 0° C., then TBDMSCl (75 mmol) and DBU (100 mmol) were successively added to the system. After the addition was complete, the reaction was left at room temperature for 12 hours. Determined by TLC that the reaction was complete, adding H 2 O (50 mL) quenched the reaction and extract...
Embodiment 2
[0234] Embodiment 2: the synthesis of methylation raw material 2b, 2u (using synthetic method two)
[0235]
[0236] Compound 2a (50 mmol) was dissolved in 50 mL of dry tetrahydrofuran, and the reaction solution was cooled to 0° C., and 1 N TBAF solution in THF (60 mL) was slowly added dropwise to the system. After the addition was complete, the reaction was left at room temperature for 12 hours. Determined by TLC that the reaction was complete, adding H 2 The reaction was quenched with O (50 mL) and extracted with ethyl acetate (3 x 100 mL). Combine the organic phases, wash with saturated NaCl solution, anhydrous Na 2 SO 4 Dry and spin off excess solvent. The crude product was separated by silica gel column chromatography (petroleum ether: ethyl acetate = 10:1-2:1) to obtain the target product 2b (14.4 g, 96%). 1 H NMR (400M Hz, CDCl 3 )δ8.55-8.52 (m, 2H), 8.13 (d, J = 7.8Hz, IH), 7.82 (td, J = 1.6, 7.7Hz, IH), 7.42-7.39 (m, 1H), 7.01-6.99 (m, 3H), 6.73(d, J=8.5Hz, ...
Embodiment 3
[0240] Embodiment 3: the synthesis of methylation raw material 2 (synthetic method three)
[0241]
[0242] Operation steps: put 2m (5mmol) in a Schlenk reaction flask, add palladium acetate (10%) and dppp (10%). After the reaction bottle was pumped and ventilated under argon protection, ROH (4.0mL), DMF (8.0mL) and triethylamine (15mmol) were added in sequence. The reaction bottle was first placed at room temperature and stirred until the raw materials were completely dissolved, and then introduced into the system Gas CO and the reaction temperature was raised to 70 ° C for 6 hours. The reaction was confirmed to be complete by TLC, the reaction was cooled to room temperature, and H 2 O (20mL) quenched the reaction, and the R"OH in the system was rotated. Extracted with ethyl acetate (3 × 30mL). The organic phases were combined, washed with saturated sodium chloride solution, anhydrous NaCl 2 SO 4 Dry and spin off excess solvent. The crude product was separated by silica...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com