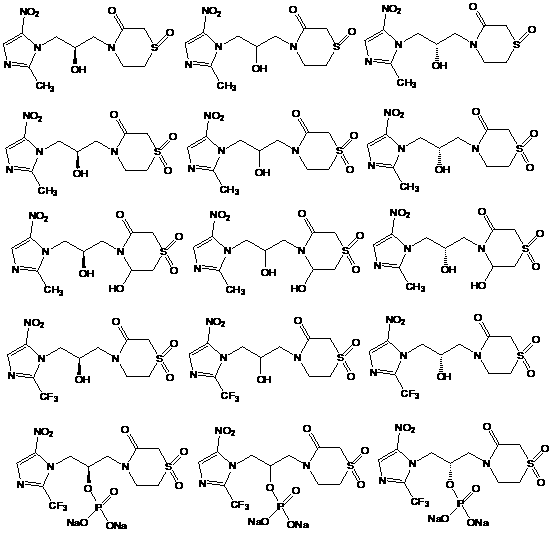
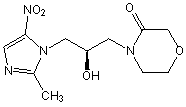
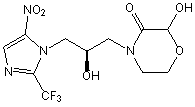
Novel nitroimidazole drug as well as preparation method and application of novel nitroimidazole drug
A technology of nitroimidazoles and drugs, which is applied in the field of new nitroimidazoles and its preparation, and can solve problems such as unfavorable drug use, sheet bleeding, stomach discomfort, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: Preparation of 2-trifluoromethylimidazole
[0035] Take 50ml of ammonia water and add it to a three-necked bottle, stir at room temperature, then take 10ml of trifluoroacetaldehyde hydrate and 10ml of glyoxal, mix them, and slowly add them dropwise to the ammonia water, react at room temperature for 2 hours, and add 20ml of ethyl acetate after the reaction is over. After two extractions for 30 min, the ethyl acetate layer was separated and dried with anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure at 60°C to obtain 8.9 g of a brown oily substance, namely 2-trifluoromethylimidazole.
Embodiment 2
[0036] Embodiment 2: Preparation of 2-methylimidazole
[0037] Take 50ml of ammonia water and add it to a three-necked bottle, stir at room temperature, then mix 10ml of acetaldehyde and 10ml of glyoxal, slowly add it dropwise to ammonia water, react at room temperature for 2 hours, after the reaction, add 20ml of ethyl acetate x 2 extractions for 30min , the ethyl acetate layer was separated and dried with anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure at 60°C to obtain 7.2 g of a brown solid, namely 2-methylimidazole.
Embodiment 3
[0038] Example 3: Preparation of 2-trifluoromethyl-5-nitroimidazole
[0039] Take 20ml of concentrated sulfuric acid and 5g of sodium sulfate into a three-necked flask, stir at room temperature, slowly add 5.0g of 2-trifluoromethylimidium, and start to heat up. After the temperature of the reaction solution reaches 150°C, slowly add 5ml of concentrated nitric acid dropwise, drop After the addition is complete, keep the reaction for 2 hours. After the reaction, cool down to 80°C and add 5ml of water, adjust the pH to 3-4 with ammonia water, a large amount of solids precipitate, filter, and dry at 60°C to obtain 2-trifluoromethyl-5-nitrate Kiimidazole 4.1g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com