Hybrid tumor cell strain 7D2 of human cardiac troponin I as well as monoclonal antibody and application thereof
A hybridoma cell line, cardiac troponin technology, applied in the field of cellular immunity, can solve problems such as deficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation of hybridoma cell line and the monoclonal antibody secreted thereof comprises the following steps:
[0025] 1. Antigen
[0026] Monoclonal antibody 7D2 is a monoclonal antibody prepared by immunizing Balb / c mice with a complete antigen coupled with amino acids 13-24 on cTnI and BSA. The preparation method of the complete antigen containing amino acids 13-24 on cTnI is as follows: : Using DiscoveryStudio4.0 software to simulate the cTnI-T-C structure, analyze the epitope on the cTnI subunit, and select the 13th-24th amino acid as the epitope antigen. The 13-24 amino acids on cTnI were synthesized and coupled with BSA to form a complete antigen. This step was synthesized by Hangzhou Zhongpei Company.
[0027] The monoclonal antibody 7H4 antibody is a monoclonal antibody prepared by immunizing Balb / c mice with the whole protein complex cTnI-T-C, wherein cTnI-T-C is purchased from Hytest Company of Finland.
[0028] 2. Preparation of hybridoma cell lines: ...
Embodiment 2
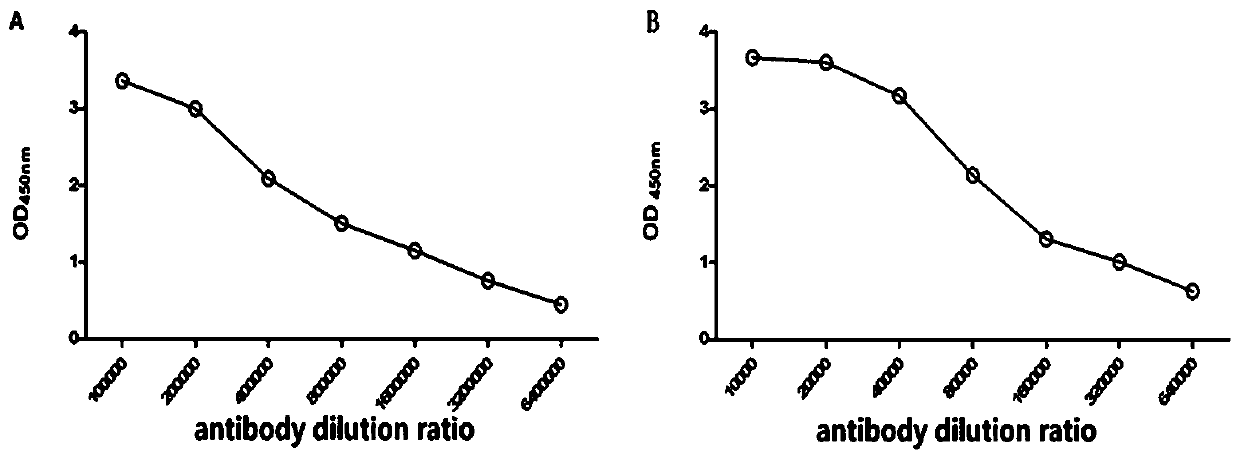
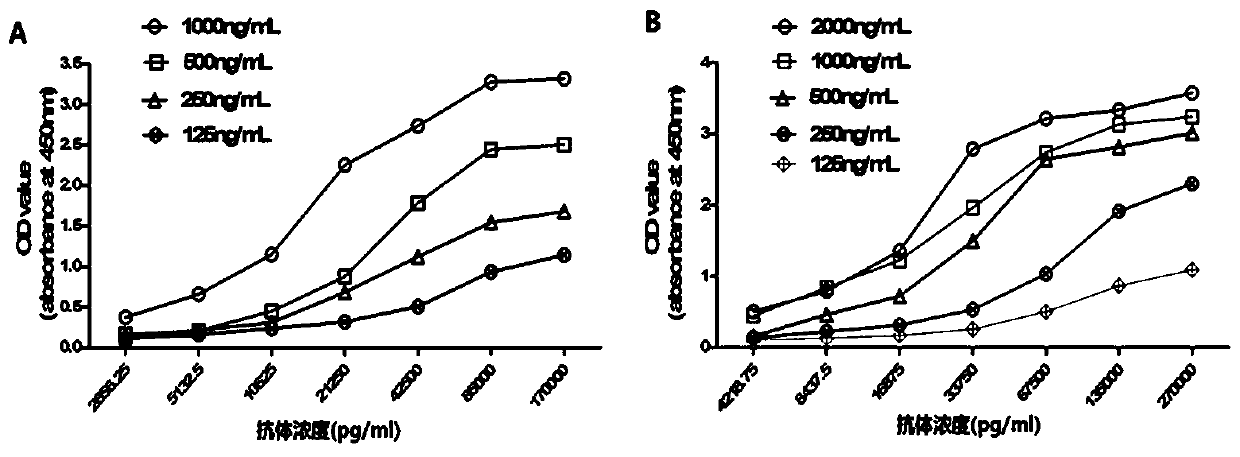
[0058] Using the monoclonal antibody 7D2 as the capture antibody and 7H4 as the detection antibody to establish a double antibody sandwich ELISA detection method, it can be used to develop a rapid detection kit for myocardial infarction. The establishment process of the double antibody sandwich ELISA method is as follows:
[0059] 1. Selection of paired antibodies
[0060] (1) Coating: Dilute antibody 7D2 to 2 μg / mL with coating solution, add 100 μL to 96-well enzyme-linked plate. overnight at 4°C. Wash three times with PBS-T buffer, 3min each time.
[0061] (2) Blocking: add 5% skimmed milk powder to the enzyme-linked plate, add 200 μL to each well, and block for one hour at 37°C. Wash three times with PBS-T buffer, 3min each time.
[0062] (3) Add antigen cTnI: Dilute the antigen to 1000ng / mL, 500ng / mL, 250ng / mL, 125ng / mL, 62.ng / mL, 31.25ng / mL, 15.625ng / mL with PBS buffer. Add 100 μL to each well. Incubate at 37°C for one hour. Wash three times with PBS-T buffer, 3min...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com