Loop-mediated isothermal amplification method implemented by combining antarctic thermal sensitive uracil deoxyribonucleic acid glycosylase (AUDG) with self-avoiding molecule recognition system (SAMRS)
A loop-mediated constant temperature and constant temperature amplification technology, applied in the field of microorganisms and molecular biology, can solve the problems of false positive results and cross-contamination, and achieve the effect of eliminating false positive results, increasing specificity and ensuring high efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1. LAMP reaction amplification
[0058] 1. Principle of LAMP reaction
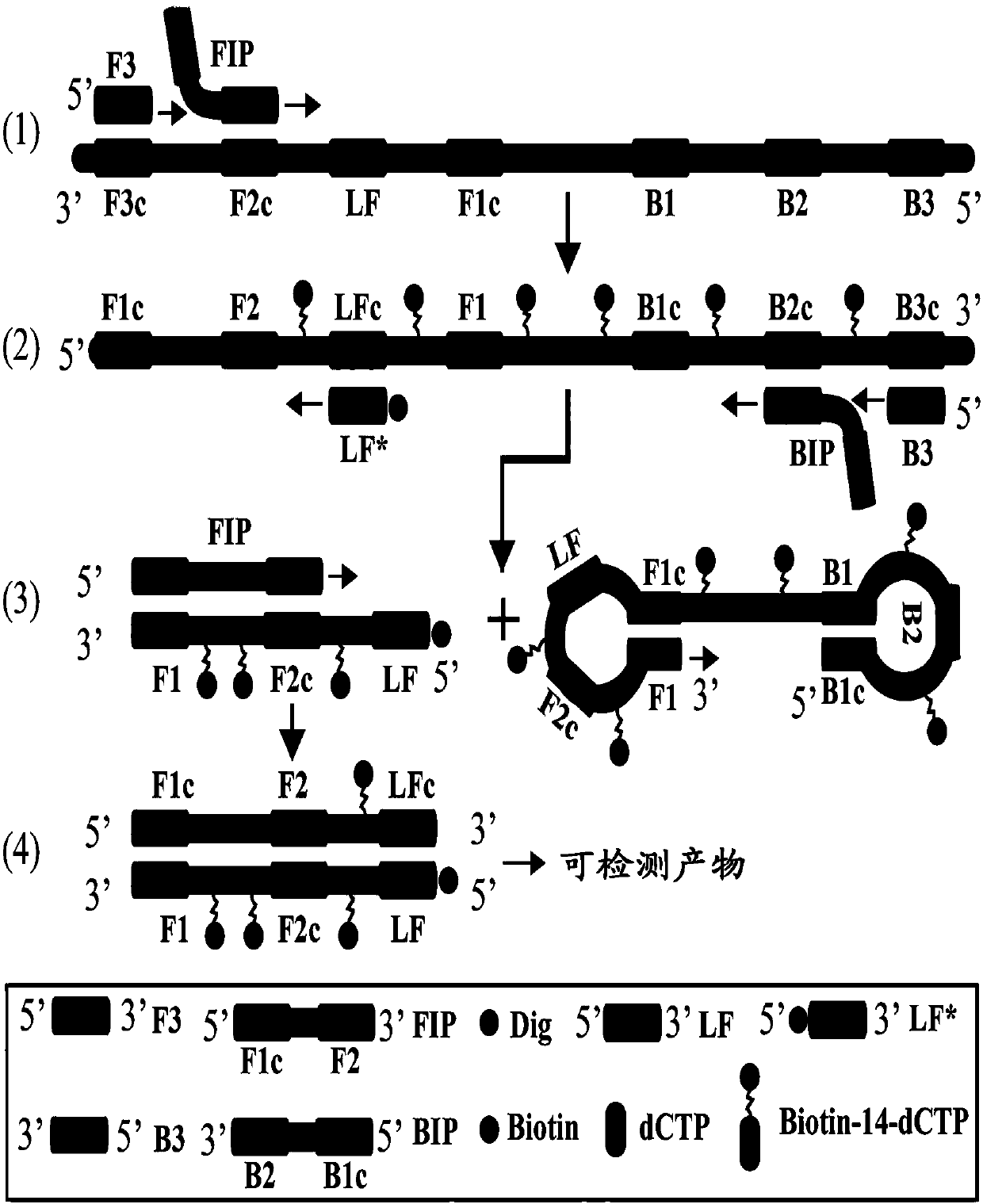
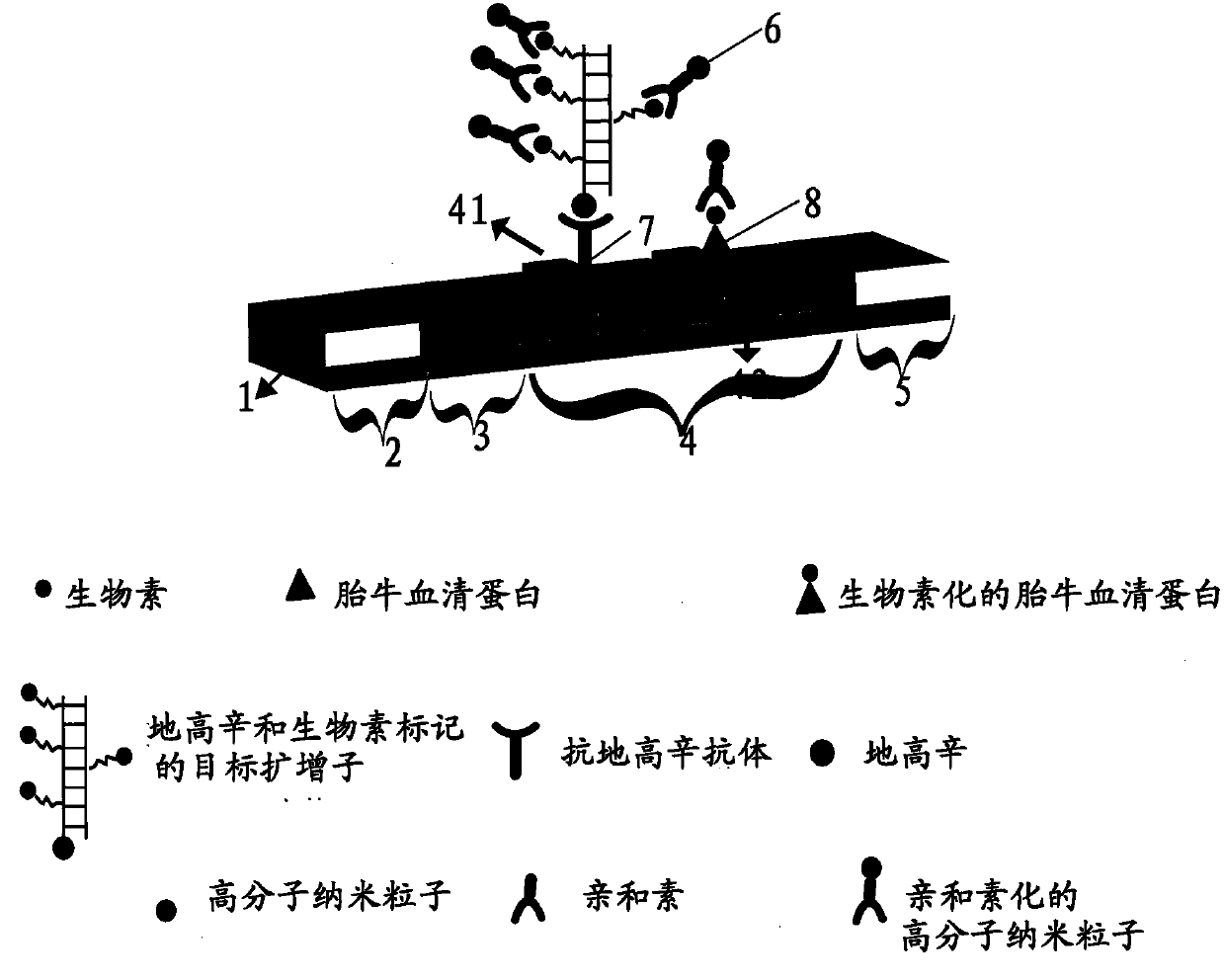
[0059] The LAMP reaction system includes 6 primers, which recognize 8 regions of the target sequence, including 2 inner primers, namely inner primers FIP and BIP; 2 replacement primers, namely F3 and B3; 2 loop primers, namely LF and LB. In order to construct a detectable product, any one of the 6 primers can be selected and labeled with a hapten (Dig, Digoxigenin) at the 5' end, and the newly labeled primers are named F3*, B3*, FIP*, BIP*, LF* and LB*. In the present invention, LF* is taken as an example to illustrate the principle of the present invention.
[0060] Under the given constant temperature conditions, the double-stranded DNA in the reaction system is in a dynamic equilibrium state of half-dissociation and half-binding. When any primer performs base pairing extension to the complementary part of the double-stranded DNA, the other strand will be dissociated. From, become a sin...
Embodiment 2
[0071] Embodiment 2.SAMRS-LAMP reaction system
[0072] 1. SAMRS-LAMP reaction principle
[0073] The reaction principle of SAMRS-LAMP is the same as that of ordinary LAMP reaction. Only the SAMRS components are used to modify the ordinary LAMP primers, which enhances the specificity of the primers, thereby enhancing the specificity of the method, thus eliminating the problems caused by off-target hybridization and primer dimers. False positive results.
[0074] 2. SAMRS-LAMP reaction system:
[0075] The concentration of SAMRS-FIP is 40pmol, the concentration of SAMRS-BIP is 40pmol, the concentration of SAMRS-F3 and SAMRS-B3 is 10pmol, the concentration of SAMRS-LF* and SAMRS-LB is 20pmol, 2M Betain, 8mM MgSO 4 , 2.5 μL of 10×Bst DNA polymerase buffer, 1.4 mM dATP, 0.7 mM dTTP, 0.7 mM dUTP, 1.38 mM dCTP, 0.02 mM biotin-14-dCTP, 1.4 mM dGTP, 10 U of chain Replace DNA polymerase, 1 μL template, add deionized water to 25 μl. The entire reaction was kept at 60°C for 1.5 hours...
Embodiment 3
[0084] Embodiment 3.AUDG-SAMRS-LAMP reaction system:
[0085] The concentration of SAMRS-FIP is 40pmol, the concentration of SAMRS-BIP is 40pmol, the concentration of SAMRS-F3 and SAMRS-B3 is 10pmol, the concentration of SAMRS-LF* and SAMRS-LB is 20pmol, 2M Betain, 8mM MgSO 4, 2.5 μL of 10×Bst DNA polymerase buffer, 1.4 mM dATP, 0.7 mM dTTP, 0.7 mM dUTP, 1.38 mM dCTP, 0.02 mM biotin-14-dCTP, 1.4 mM dGTP, 10 U of chain Replace DNA polymerase, 1U Antarctic thermosensitive uracil deoxynucleic acid glycosylase, 1 μL template, add deionized water to 25 μl. The entire reaction was kept at 60°C for 1.5 hours, and the reaction was terminated at 80°C for 5 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com