Pharmaceutical composition for treating hepatitis, pharmaceutical preparation as well as application and preparation method thereof
A composition and medicine technology, applied in the field of pharmaceutical preparations for treating hepatitis, can solve the problems of unclear active ingredients and their mechanism of action, large dosage and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
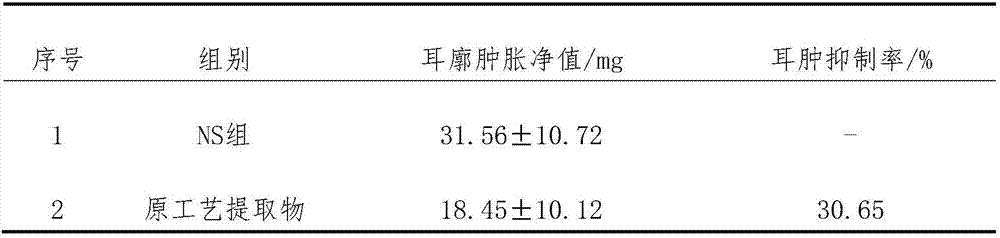
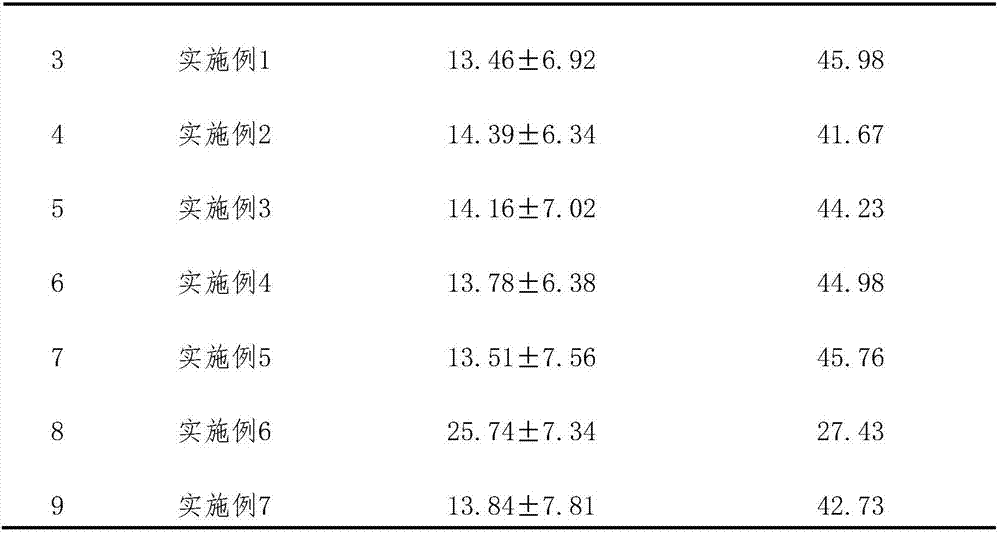
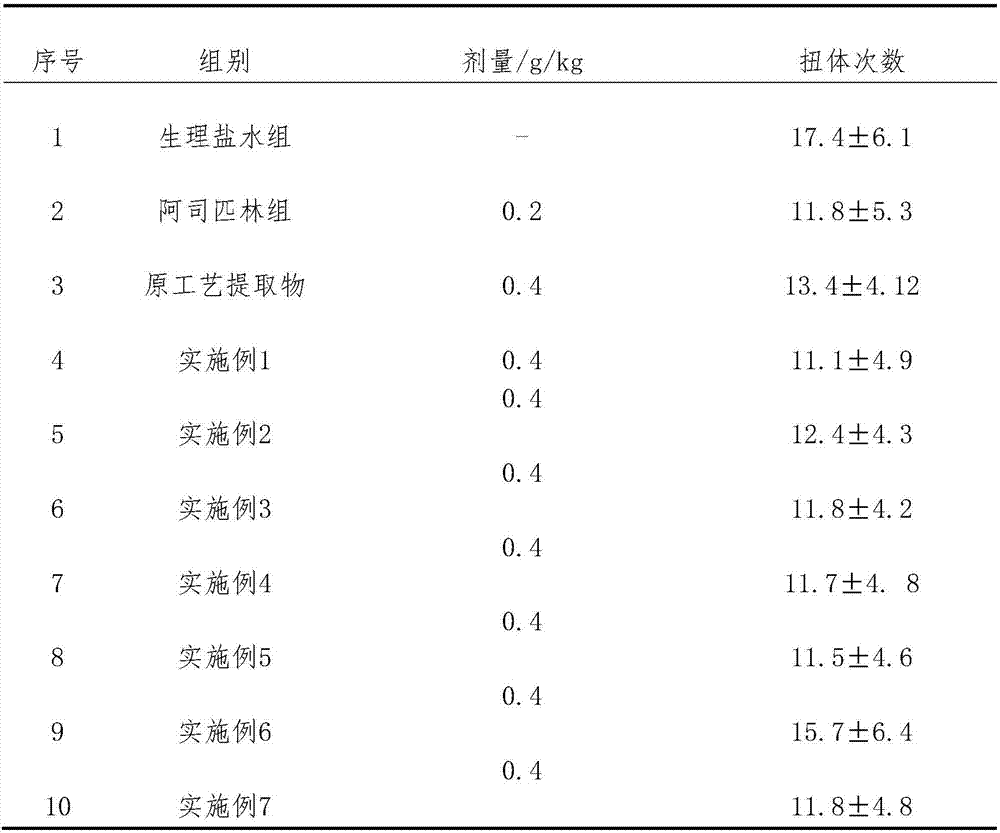
[0045] Embodiment 1: the preparation that has sugar type tablet
[0046] Take 8.45g of total flavonoids of horseshoe gold, 2.56g of quercetin, 1.91g of verbenin, 8.96g of tetrandrine, 7.62g of tetrandrine, 1.42g of hedera saponin, 2.82g of icariin, and 0.70g of astragaloside g, 15.76g of paeoniflorin, 5.54g of tanshinone IIA, 4.15g of cryptotanshinone, add 12 times of 75% ethanol solution, stir and dissolve, place for 48h, filter, recover ethanol from the filtrate and concentrate to a relative density of 1.37 (55°C) thick paste, add 32% starch and 1.3% microcrystalline cellulose, granulate, dry at 60°C, add 0.1% magnesium stearate, mix, tablet, film-coat, 1000 tablets were prepared, each with about 59.98 mg of active ingredient.
Embodiment 2
[0047] Embodiment 2: Preparation of sugar-free granules
[0048] Take 7.82g of total flavonoids of horseshoe gold, 2.37g of quercetin, 1.66g of verbenin, 6.51g of tetrandrine, 6.06g of tetrandrine, 1.20g of hedera saponin, 2.56g of icariin, and 0.62g of astragaloside g, paeoniflorin 14.30g, tanshinone ⅡA 4.83g, cryptotanshinone 3.42g, add 10 times of 70% ethanol solution, stir and dissolve, let stand for 48h, filter, recover ethanol from the filtrate and concentrate to a relative density of 1.34 (50°C) Add 60% dextrin to make sugar-free granules, spray 80ml of 2% hydroxypropyl methylcellulose ethanol solution into granules after the granules are formed, and make 1000 bags of granules, each bag Active ingredient approx. 51.35 mg.
Embodiment 3
[0049] Embodiment 3: the preparation of capsule
[0050] Take 9.00g of total flavonoids of horseshoe gold, 2.71g of quercetin, 2.15g of verbenin, 6.51g of tetrandrine, 9.83g of tetrandrine, 1.20g of hedera saponin, 3.17g of icariin, and 0.62g of astragaloside g, paeoniflorin 17.88g, tanshinone ⅡA 4.83g, cryptotanshinone 4.68g, add 10 times of 80% ethanol solution, stir and dissolve, put it aside for 48h, filter, recover ethanol from the filtrate and concentrate to a relative density of 1.34 (60°C) The thick paste is dried at 60° C., pulverized, added with 0.5% magnesium stearate, mixed evenly, and packed into capsules to obtain 1000 capsules, each with about 62.58 mg of active ingredient.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com