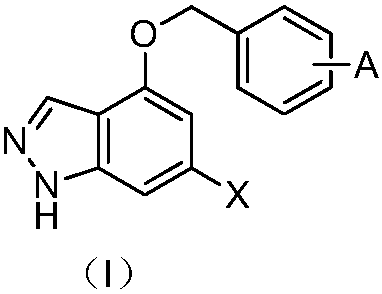
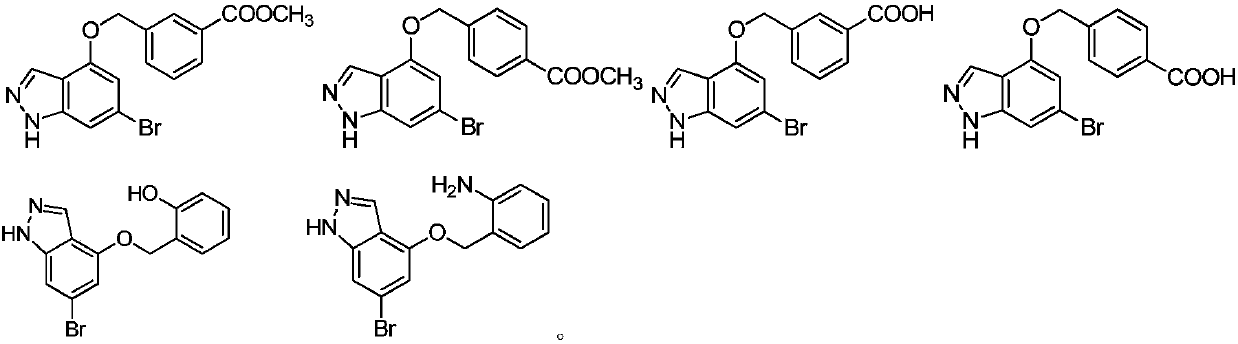
1H-indazole-4-ether compound and application of 1H-indazole-4-ether compound serving as IDO inhibitor
A compound and solvate technology, applied in the preparation of carbon-based compounds, amino compounds, and organic compounds, etc., can solve the problems of inhibiting killing effect, stagnation of synthesis, and reducing the concentration of tryptophan.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] The preparation of embodiment 1 compound of the present invention
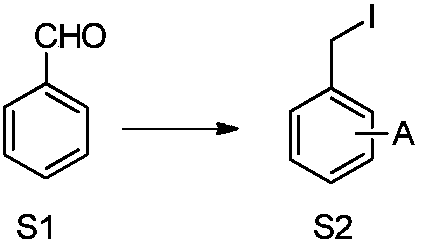
[0076] 1. Synthesis of key intermediates
[0077] 1, the synthesis of methyl m-iodomethylbenzoate (5)
[0078]
[0079] Compound 1 (1.00g, 6.67mmol) was dissolved in 15mL of methanol, and SOCl was added under stirring at 0°C 2 (1.45mL, 20.00mmol), warmed up to room temperature and stirred for 12h, then the reaction was complete by TCL detection. The reaction solution was concentrated, water was added to the residue, and saturated NaHCO 3 The aqueous solution was adjusted to pH=7-8. Extracted 3 times with ethyl acetate. The organic layer was washed with water, dried, and concentrated to obtain compound 3 (0.83 g, yield 76%) as a pale yellow oil. It was directly used in the next step of synthesis without purification by column chromatography.
[0080] Compound 3 (1.00g, 6.09mmol) and I 2 (0.93g, 3.67mmol) dissolved in 10mL CH 2 Cl 2 , cooled to -5°C and stirred. Dissolve 1,1,3,3-tetramethyldi...
Embodiment 2
[0122] Embodiment 2 The inhibitory activity of the compound of the present invention to IDO protein
[0123] Recombinant human IDO protein is expressed in Escherichia coli and purified by nickel affinity chromatography. The compound's IDO inhibitory activity test uses L-tryptophan as a substrate. The compounds to be tested were dissolved in 10% DMSO solution to prepare dilutions. Take 5uL of the diluted solution and add it to the 100μL reaction system. The 100 μL reaction system contained 0.5% DMSO, 40 nmol / L IDO, 900 μmol / LL-tryptophan, and other reaction coexistents (potassium phosphate buffer, ascorbic acid, catalase, methylene blue). The reaction mixture was incubated at 37°C for 180 minutes, and then trichloroacetic acid was added to terminate the reaction. The concentration of N-formylkynurenine produced was measured at 321 nm using a Tecan Infinite M1000 microplate reader to evaluate the inhibitory activity of the compound on IDO. The negative control was 5 μL of bu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com