Catalyst for catalytic oxidation of halogenated hydrocarbon and preparation method thereof
A catalytic oxidation and catalyst technology, which is applied to the catalyst for catalytic oxidation of halogenated hydrocarbons and the field of preparation thereof, can solve the problems of high decomposition temperature of chlorine-containing alkanes, complex preparation process, low catalytic efficiency, etc., and achieves improved catalyst performance and simple preparation process. , the effect of low cost of preparation of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
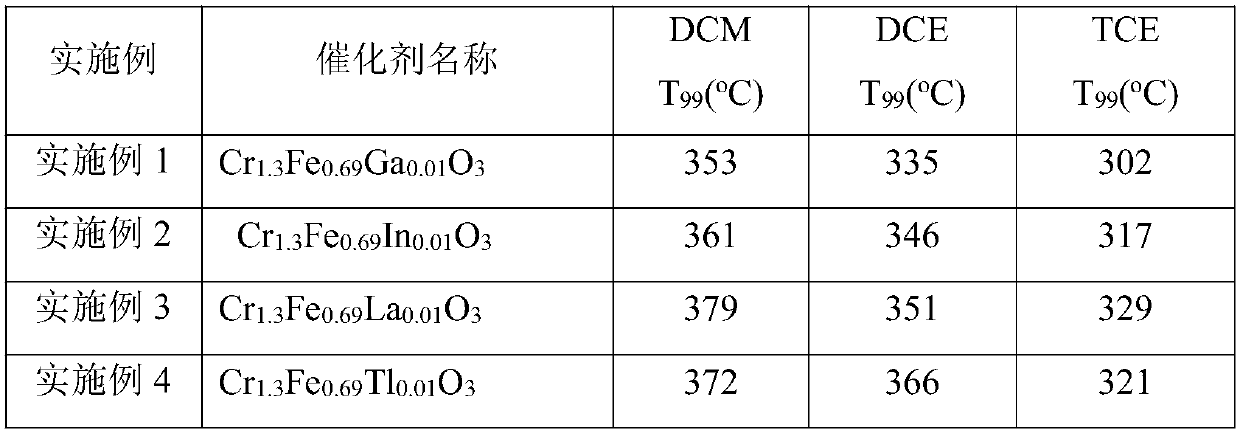
[0013] ①Ga(NO 3 ) 3 ·8H 2 O, Fe(NO 3 ) 3 9H 2 O, Cr(NO 3 ) 3 9H 2 O and citric acid were weighed in Ga(NO 3 ) 3 ·8H 2 O 0.04g, Fe(NO 3 ) 3 9H 2 O 2.79g, Cr(NO 3 ) 3 9H 2 O5.20g, citric acid 8.40g, add 100ml deionized water, stir in 50°C water bath for 30min to dissolve. Then the above mixture was continuously stirred at 90 °C for 4 h to form a brown gel. Cool at room temperature, dry at 120°C for 12h, and finally bake at 600°C for 4h in an air atmosphere to obtain the catalyst carrier Cr of the present invention. 1.3 Fe 0.69 Ga 0.01 o 3 .
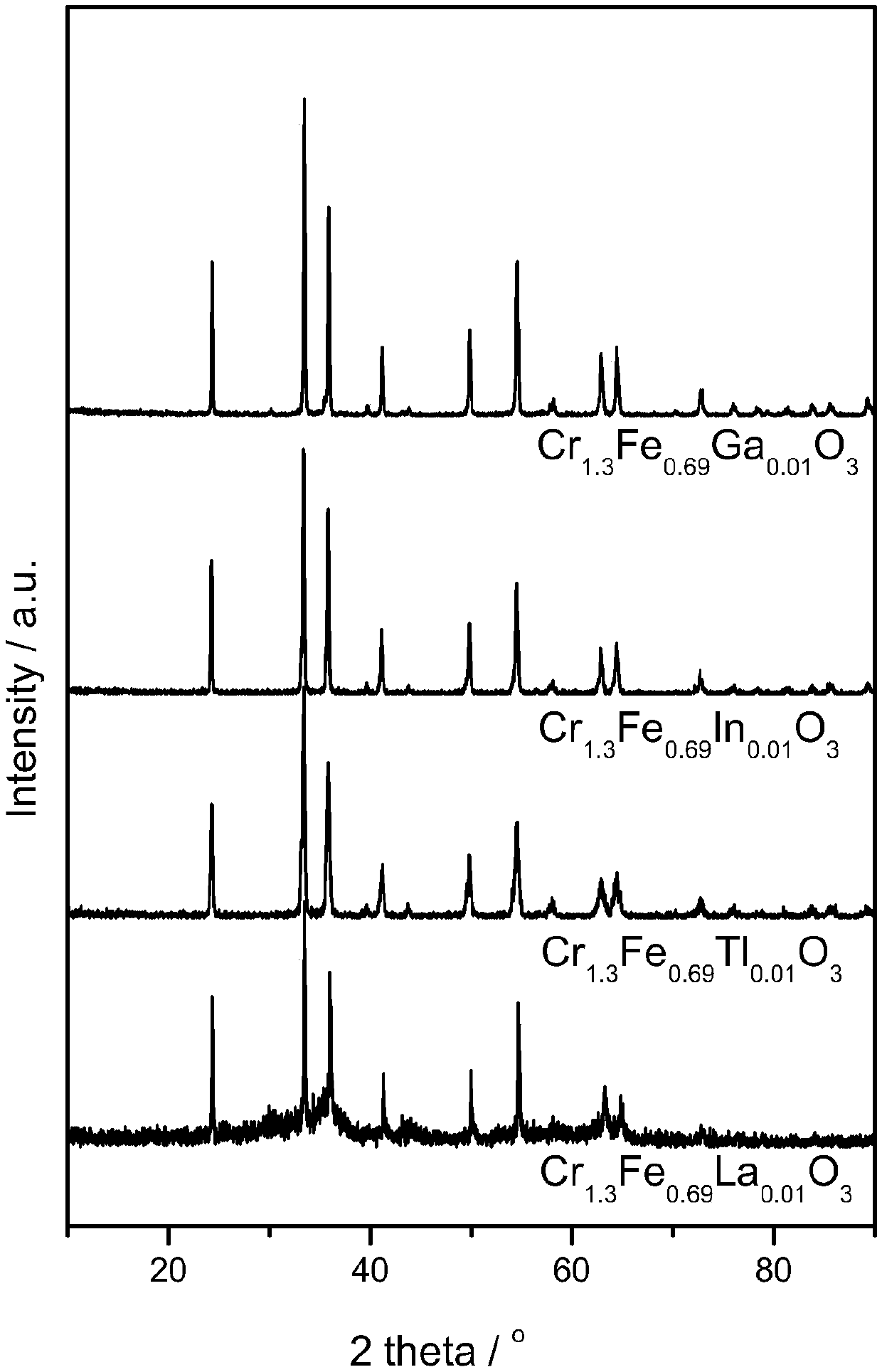
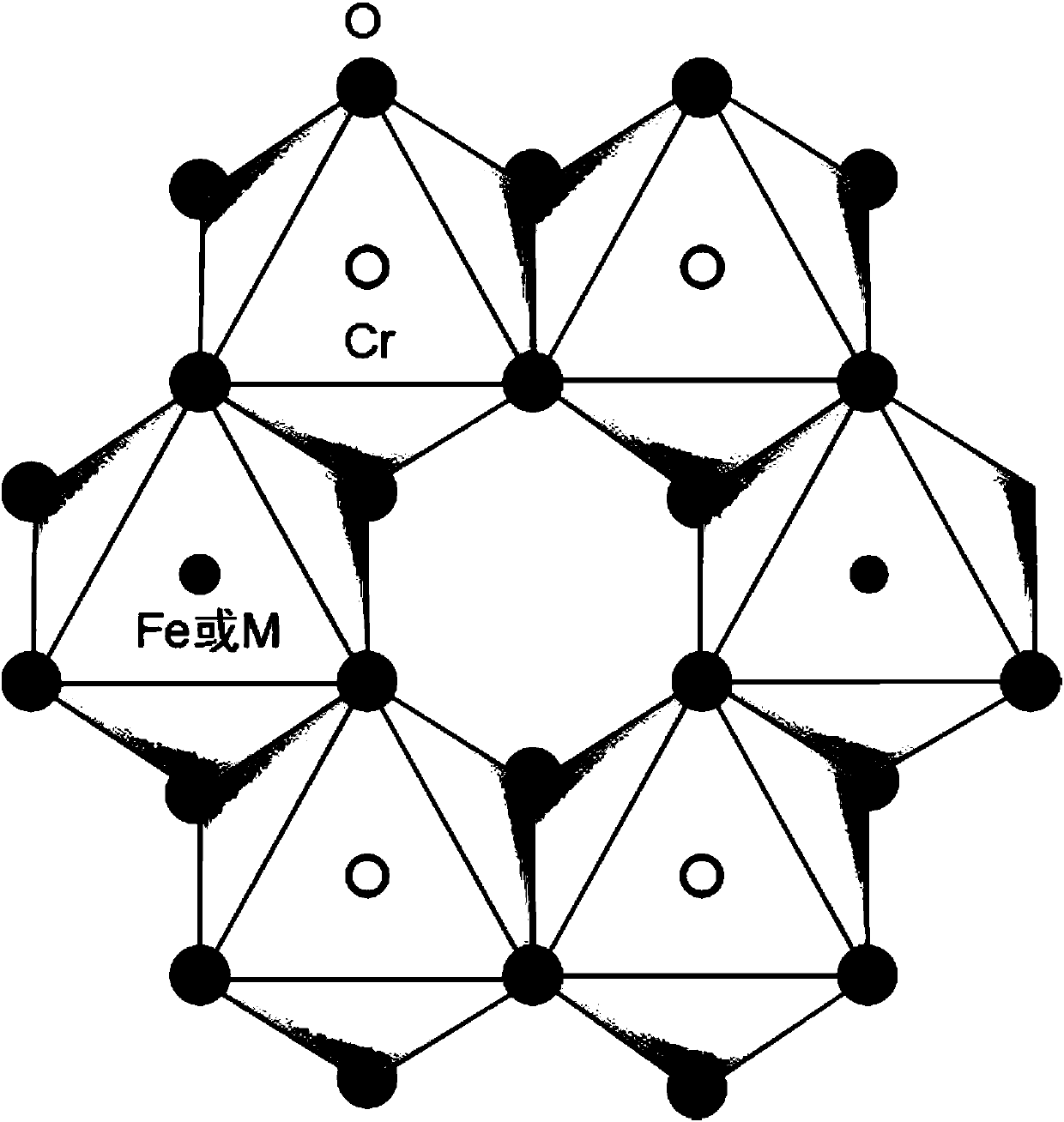
[0014] ② The structure of the catalyst carrier is shown in the attached figure. figure 1 for Cr 1.3 Fe 0.69 m 0.01 o 3 (M=Ga, In, Tl or La) catalyst XRD pattern, after comparing the XRD standard card, the crystal phase of the synthesized compound is highly consistent with the standard card PDF-35-1112, indicating that the synthesized sample is Cr with corundum structure 1.3 Fe 0.7 o 3 type of compound, and there...
Embodiment 2
[0017] ①In(NO 3 ) 3 ·6H 2 O, Fe(NO 3 ) 3 9H 2 O, Cr(NO 3 ) 3 9H 2 O and citric acid according to the molar ratio of 0.01:0.69:1.3:4, weigh In(NO 3 ) 3 ·6H 2 O 0.04g, Fe(NO3 ) 3 9H 2 O 2.79g, Cr(NO 3 ) 3 9H 2 O5.20g, citric acid 8.40g, add 100ml deionized water, stir in 50°C water bath for 30min to dissolve. Then the above mixture was continuously stirred at 90 °C for 4 h to form a brown gel. Cool at room temperature, dry at 120°C for 12h, and finally bake at 600°C for 4h in an air atmosphere to obtain the catalyst carrier Cr of the present invention. 1.3 Fe 0.69 In 0.01 o 3 .
[0018] ② The structure of the catalyst carrier is the same as in Example 1.
[0019] ③ The catalyst support activity evaluation conditions are the same as in Example 1.
Embodiment 3
[0021] ①La(NO 3 ) 3 ·6H 2 O, Fe(NO 3 ) 3 9H 2 O, Cr(NO 3 ) 3 9H 2 According to the molar ratio of O and citric acid, La(NO 3 ) 3 ·6H 2 O 0.04g, Fe(NO 3 ) 3 9H 2 O 2.79g, Cr(NO 3 ) 3 9H 2 O5.20g, citric acid 8.40g, add 100ml deionized water, stir in 50°C water bath for 30min to dissolve. Then the above mixture was continuously stirred at 90 °C for 4 h to form a brown gel. Cool at room temperature, dry at 120°C for 12h, and finally bake at 600°C for 4h in an air atmosphere to obtain the catalyst carrier Cr of the present invention. 1.3 Fe 0.69 La 0.01 o 3 .
[0022] ② The structure of the catalyst carrier is the same as in Example 1.
[0023] ③ The catalyst support activity evaluation conditions are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com