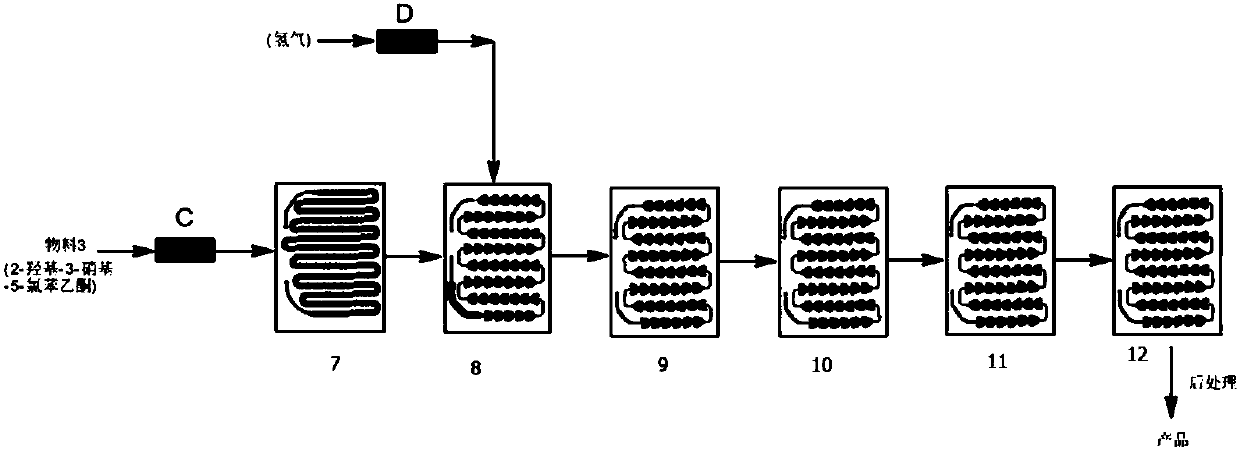
Synthesis method of 3-amino-2-hydroxyacetophenone
A technology of hydroxyacetophenone and a synthesis method, which is applied in the preparation of nitro compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of low number of times of catalyst recovery, long reaction time, explosion safety, etc., and achieve control High temperature degradation of impurity generation, long reaction time and high space-time efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
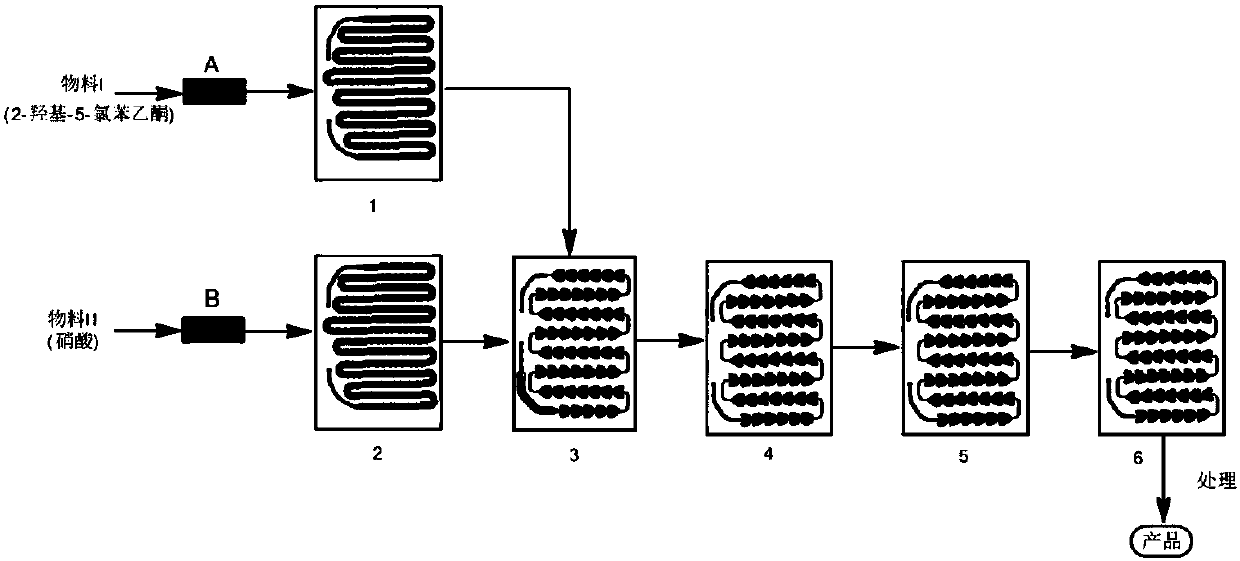
[0045] Weigh 180.00g of 2-hydroxy-5-chloroacetophenone and add it to 1200ml of glacial acetic acid to form material I after stirring and dissolving, weigh 100g of fuming nitric acid and add it to 800ml of glacial acetic acid to form material II, and adjust the flow rate of metering pump A Make the flow velocity of material I be 15ml / min, the flow velocity of regulating metering pump B makes the flow velocity of material II be 7ml / min, temperature of reaction is 65 ℃, and the mol ratio of nitric acid and 2-hydroxyl-5-chloroacetophenone is 1.5: 1. The residence time of the reaction is 95 seconds. Collect the reaction solution flowing out from the outlet, measure 3600ml of water and slowly drop it into the reaction system, a large amount of yellow solid precipitates, keep stirring at room temperature for 1h, filter, and add 500ml of cold ethanol to the filter cake to wash. Vacuum drying at 50°C for 6 hours gave 207.76 g of 2-hydroxy-3-nitro-5-chloroacetophenone with a yield of 91....
Embodiment 2
[0048] Weigh 150.00g of 2-hydroxyl-5-chloroacetophenone and add it into 1000ml of glacial acetic acid to form material I after stirring and dissolving; take 92g of found nitric acid and add it into 500ml of glacial acetic acid to form material II; adjust the flow rate of metering pump A to The flow rate of material I is 18ml / min, adjust the flow rate of metering pump B so that the flow rate of material II is 9ml / min, the reaction temperature is 60°C, and the molar ratio of nitric acid to 2-hydroxy-5-chloroacetophenone is 1.5:1 , the residence time of the reaction is 75 seconds, collect the reaction solution flowing out from the outlet, measure 3000ml of water and slowly drop it into the reaction system, a large amount of yellow solid is precipitated, stirred at room temperature for 1h, filtered, and the filter cake was washed with 450ml of cold ethanol, 50 Vacuum drying at °C for 6 hours gave 171.73 g of 2-hydroxy-3-nitro-5-chloroacetophenone with a yield of 90.59% and a purity...
Embodiment 3
[0051] Weigh 200.00g of 2-hydroxy-5-chloroacetophenone and add it to 1600ml of glacial acetic acid to form material I after stirring and dissolving. Weigh 100g of fuming nitric acid and add it to 800ml of glacial acetic acid to form material II. Adjust the flow rate of metering pump A Make the flow velocity of material I be 12ml / min, the flow velocity of regulating metering pump B makes the flow velocity of material II be 6ml / min, temperature of reaction is 70 ℃, and the mol ratio of nitric acid and 2-hydroxyl-5-chloroacetophenone is 1.2: 1. The residence time of the reaction is 100 seconds. Collect the reaction solution flowing out from the outlet, measure 4800ml of water and slowly drop it into the reaction system, a large amount of yellow solids will precipitate, keep stirring at room temperature for 1 hour, filter, and add 600ml of cold ethanol to the filter cake to wash. Vacuum drying at 50°C for 6 hours gave 229.63 g of 2-hydroxy-3-nitro-5-chloroacetophenone with a yield ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com