Application of neuraminidase and inhibitor to preparation of medicines for treating hypertension
A technology of neuraminidase and high blood pressure, which is applied in the field of biomedicine and can solve problems such as the undiscovered correlation of neuraminidase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 1. Experimental method
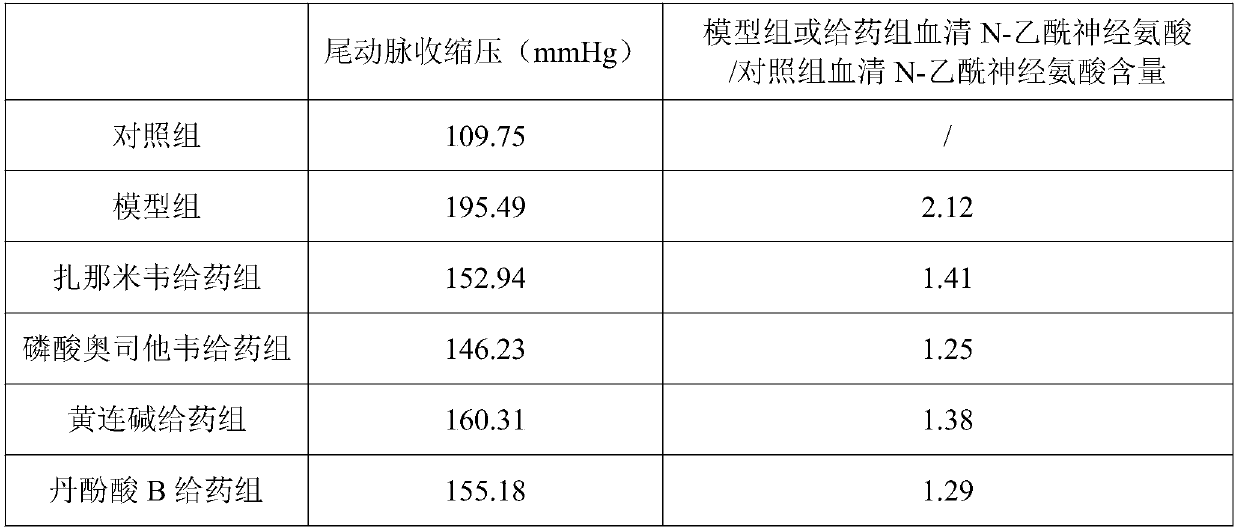
[0019] SPF grade 9-week-old SHR male rats (body weight 180-200g) were fed freely for 3 days, and their blood pressure was measured 5 times adaptively. Divided into 5 groups, 10 rats in each group: model group and zanamivir, oseltamivir phosphate, coptisine, salvianolic acid B administration group. Another 9-week-old normotensive WKY male rats were used as the control group. Rats in the administration group were given 0.2mg / kg / d zanamivir (i.v.), 5mg / kg / d oseltamivir phosphate (p.o.), 40mg / kg / d coptisine (p.o.), 40mg / kg / d salvianolic acid B (p.o.), the model group and the control group were administered intragastrically with an equal volume of 0.5% CMC-Na, and the administration continued for 4 weeks.
[0020] After 4 weeks of administration, operate according to the requirements of the automatic non-invasive blood pressure measurement system (Chengdu Taimeng; model BP-600A), and measure the blood pressure of each group of rats 1 hour after th...
Embodiment 2
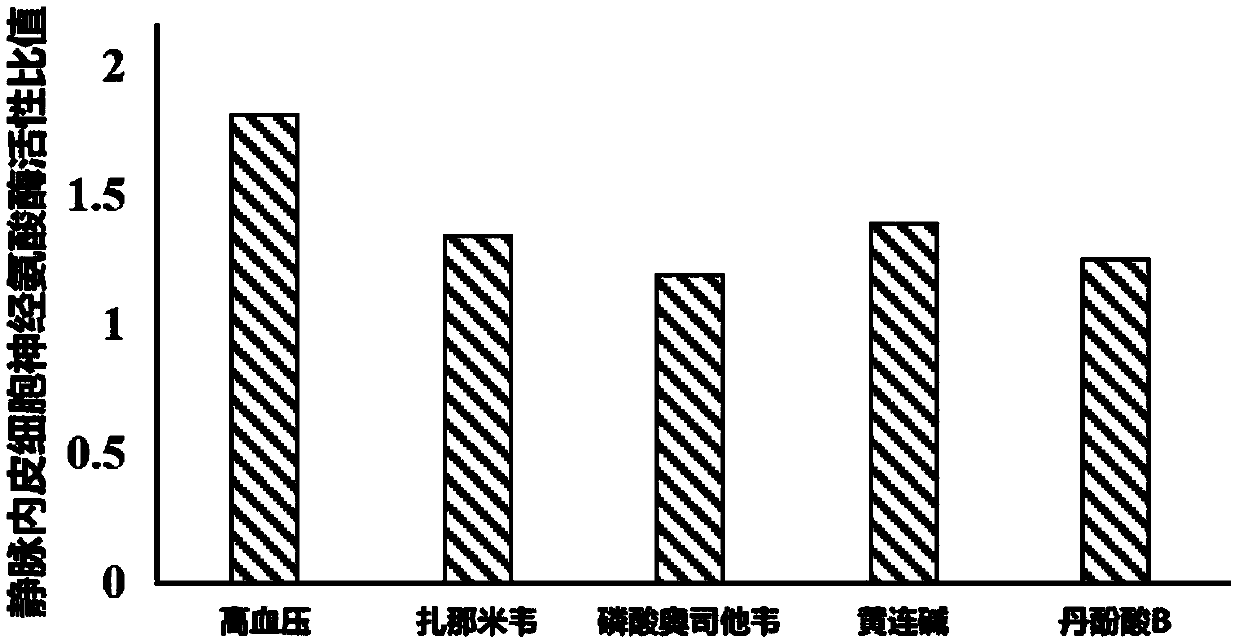
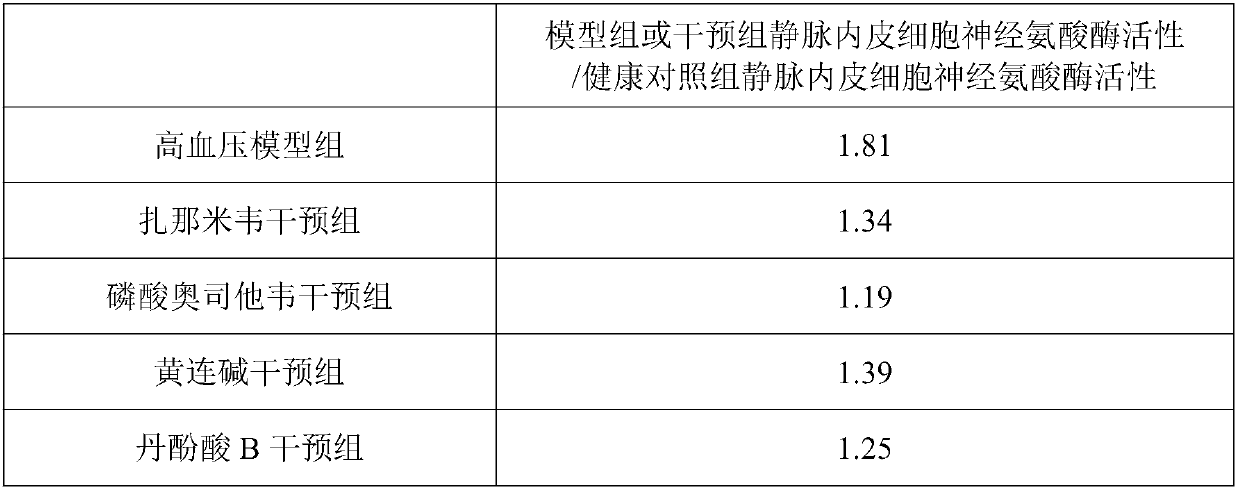
[0027] The human umbilical vein endothelial cell line (HUVEC-c) was cultured in high-glucose DMEM medium with 10% fetal bovine serum and 1% double antibody at 37°C and 5% CO 2 . The medium was changed every other day, after being digested and passaged with 0.25% trypsin, they were replanted in culture dishes according to different conditional media, and were divided into blank control group (control group), hypertension model group (hypertension group), zanami Zanamivir intervention group (zanamivir group), oseltamivir phosphate intervention group (oseltamivir phosphate group), coptisine intervention group (coptisine group), salvianolic acid B intervention group (salvianolic acid B group) .
[0028] The conditioned medium was prepared according to the experimental groups as follows:
[0029] ①Blank control group: normal medium containing 10% serum; ②Hypertensive model group: normal medium containing 10% serum and angiotensin II at a concentration of 1 μg / ml; ③Zanamivir inter...
Embodiment 3
[0036] The commercially available neuraminidase inhibitor screening kit P0309 (Beyotime, Beyotime) was used to test the inhibitory activity of salvianolic acid B in vitro, and the positive control drug was oseltamivir phosphate. Add 70 μL of buffer solution and 10 μL of neuraminidase solution to each well of a 96-well plate, then add 10 μL of different concentrations of the test solution, shake and mix, incubate at 37°C for 5 minutes, add 10 μL of the solution containing the fluorescent substrate, shake and mix Homogenize, incubate at 37°C for 30min, and perform fluorescence measurement, wherein the excitation wavelength is 322nm and the emission wavelength is 450nm. The inhibition rates of different test solutions were calculated according to the fluorescence readings, and the IC50 values of the positive control drugs oseltamivir phosphate and salvianolic acid B were further obtained. The results are shown in Table 3.
[0037] Table 3 IC50 values of positive control drug...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com