Targeted anti-tumor drug system and preparation method thereof
An anti-tumor drug and targeting technology, applied in anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., can solve the problems of inability to distinguish between normal cells and cancer cells, lack of targeting, hair loss, etc., to achieve dual inhibition of tumor growth, The effect of increasing biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The preparation process of the annealing solution is as follows: take 200mM KCl, 4mM MgCl 2 , 28mM Tris-HCl in a container, dissolved in water, and quantitatively prepared to 1L.
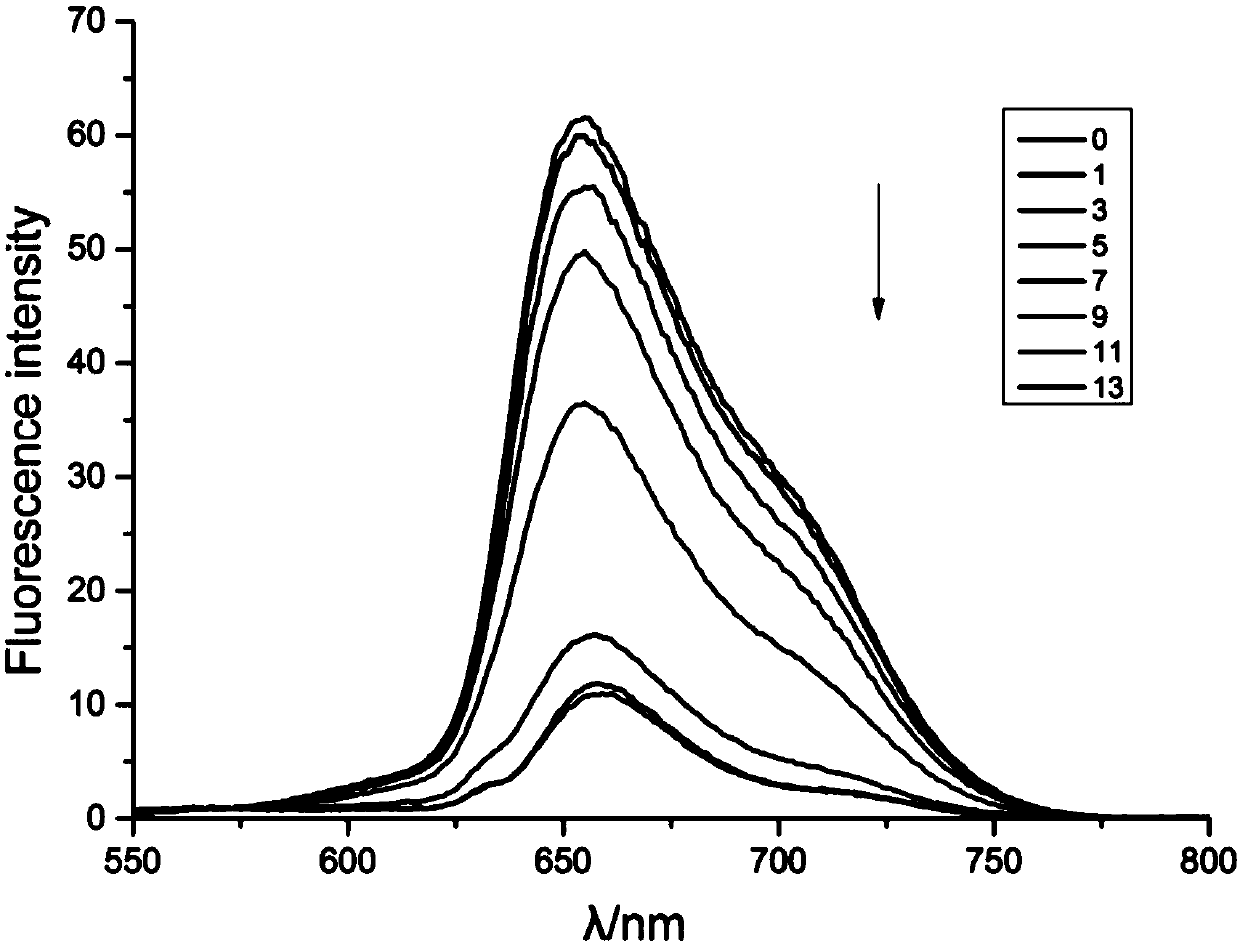
[0043] Both DNM and TMPYP have certain fluorescence. When combined with DNA, the fluorescence is quenched. This characteristic was used to determine the immobilization of APS8 to DNM and TMPYP.
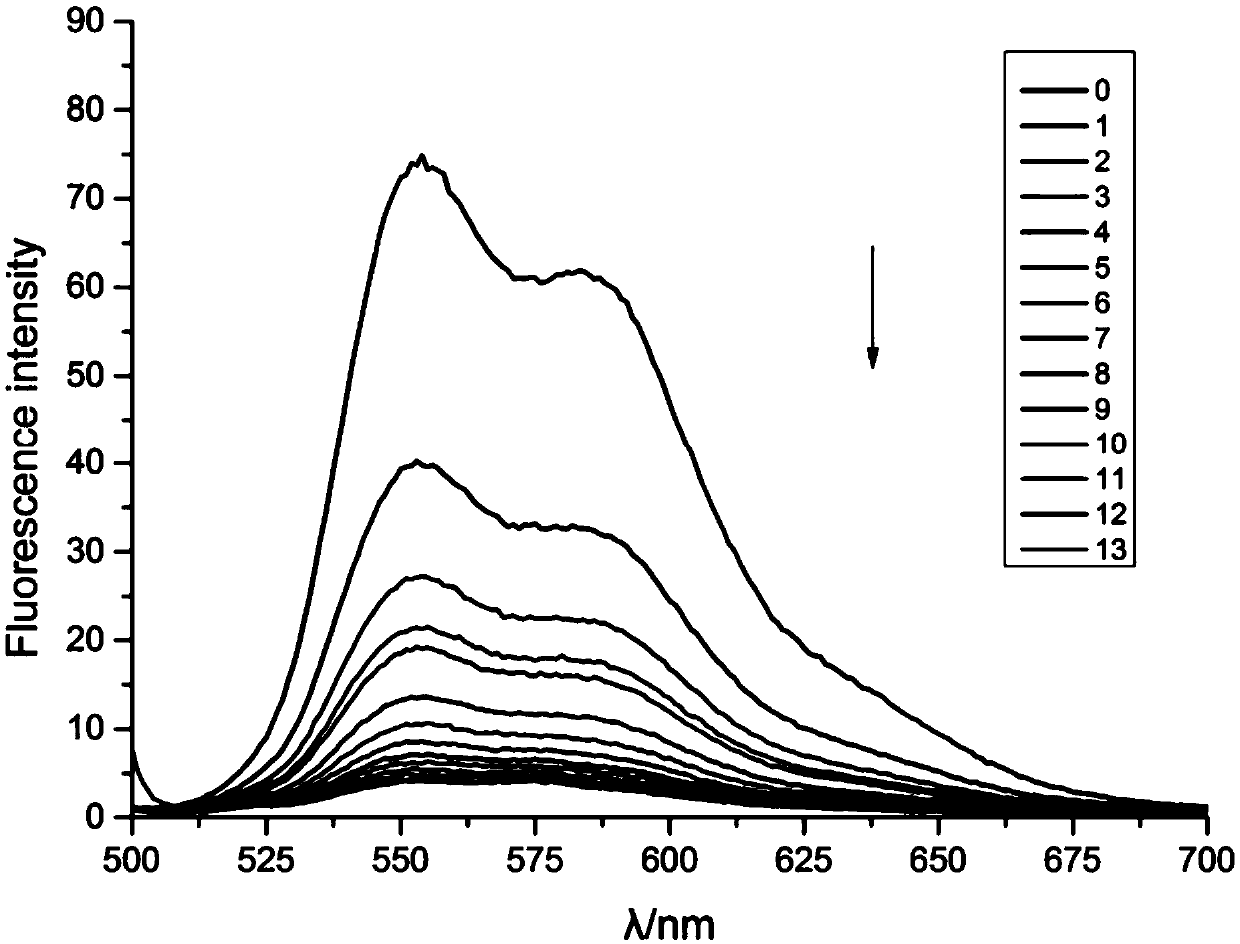
[0044] Dissolve 10 μl of DNM with a concentration of 189.566 μmol / l in 2.5 ml of ultrapure water, measure its fluorescence with a fluorescence spectrophotometer, add 1 μl of annealed APS8 with a concentration of 50 μmol / l in turn, and wait for 5 minutes after fully mixing and reacting. Fluorescence changes were measured, and the results of fluorescence detection were as follows: figure 1 as shown, figure 1The direction of the middle arrow indicates the change of fluorescence with the concentration of APS8 increasing gradually, and the unit of the label number is μl. The fluorescence condition of ...
Embodiment 1
[0046] Example 1 Preparation of targeted anti-tumor drug system APS8@DNM@TMPYP
[0047] 1 drug immobilization
[0048] According to the molar ratio of APS8: DNM: TMPYP = 1:1:0.5 immobilized:
[0049] Immobilized DNM: Take 25 μl of single-chain APS8 solution, add 10.1 μl of annealing solution and mix well, anneal at 90°C for 10 minutes on the PCR instrument, and slowly cool down to room temperature. Take out the annealed APS8 solution, add 13.18 μl DNM (189.566 μmol / l), mix with a mixer, and immobilize it in an ice bath for 2 hours to obtain APS8@DNM;
[0050] Immobilized TMPYP: Take out the APS8 (APS8@DNM) solution that has been immobilized with DNM, add 1.7 μl of TMPYP solution (733.35 μmol / l), and immobilize it at room temperature for 2 hours to obtain the targeted anti-tumor drug system APS8@DNM@TMPYP, The specific process is as Figure 8 As shown, the final concentration of DNM in the targeted anti-tumor drug system is 50 μmol / l, the final concentration of TMPYP is 25 μ...
Embodiment 2
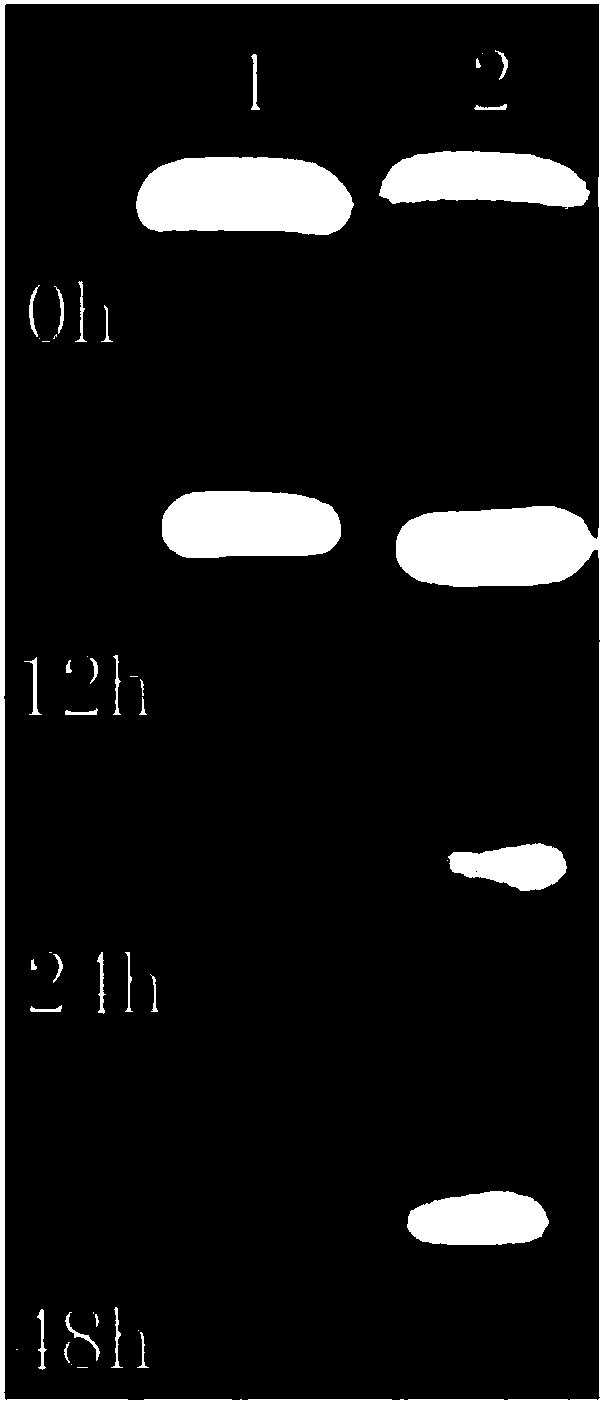
[0051] Example 2 Stability investigation of targeted anti-tumor drug system APS8@DNM@TMPYP
[0052] The APS8@DNM@TMPYP nano-drug delivery system is mainly used for drug delivery and targeted therapy of tumor cells, so the stability of the entire composite system in serum needs to be investigated.
[0053] Methods as below:
[0054] 10XTBE stock solution: 108g of Tris Base (2-amino-2-(hydroxymethyl)-1,3-propanediol), 9.2g of EDTA, 55.2g of boric acid in a container, add water to dissolve, adjust the pH to 8.3, quantitatively to 1L.
[0055] 1XTBE electrophoresis buffer: take 100ml of 10XTBE stock solution, add water to make up to 1L.
[0056] Agarose gel: First prepare 1% agarose gel, weigh 0.15g of agarose and add it to 15ml of 1XTBE buffer, heat to dissolve into a transparent solution, and add 2μl of nucleic acid staining solution after the temperature drops to about 60°C. Shake well and pour into the plastic plate, let stand for 20 minutes.
[0057] Take 27 μl each of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com