Preparation method of environment-friendly quinoline compound
A compound, quinoline technology, which is applied in pharmaceutical and chemical intermediates and related chemical fields, can solve problems such as limitations, and achieve the effects of simple operation, environmental friendliness, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
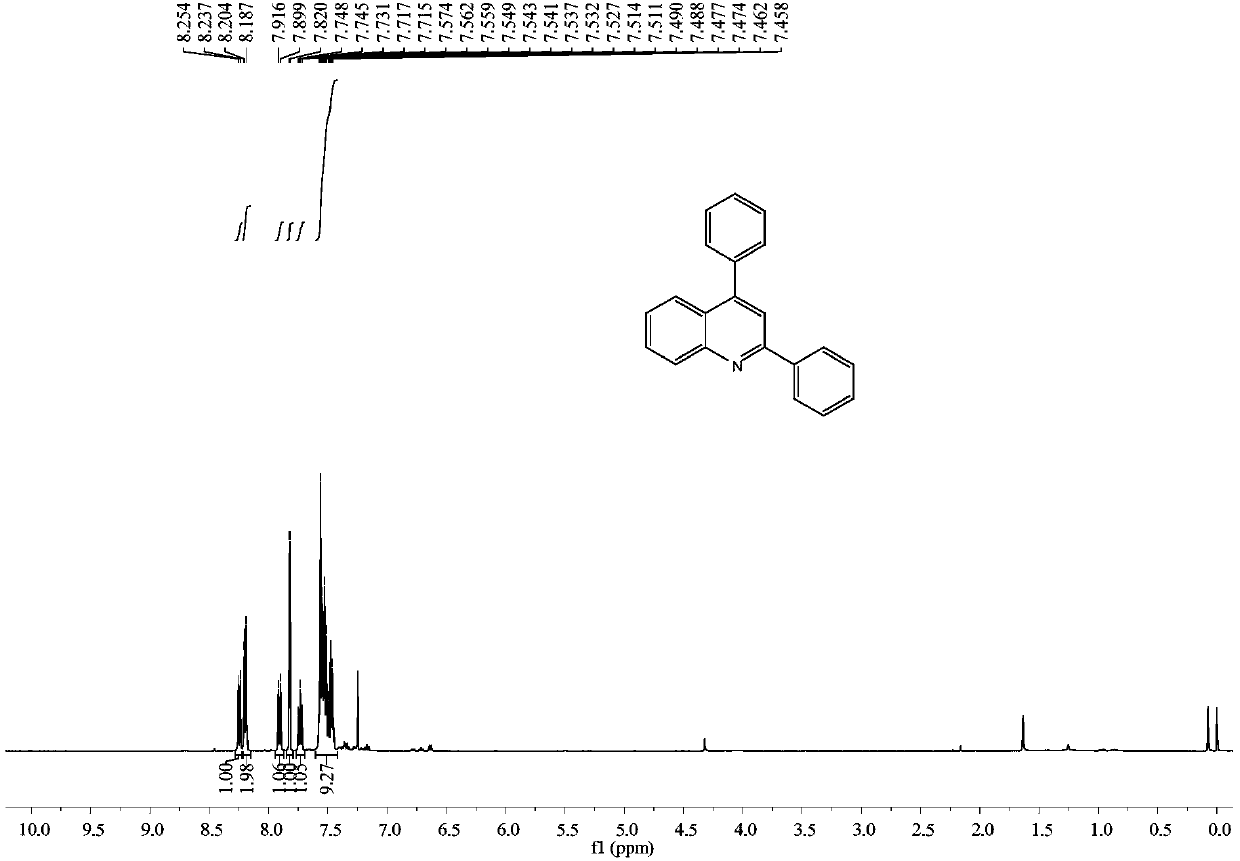
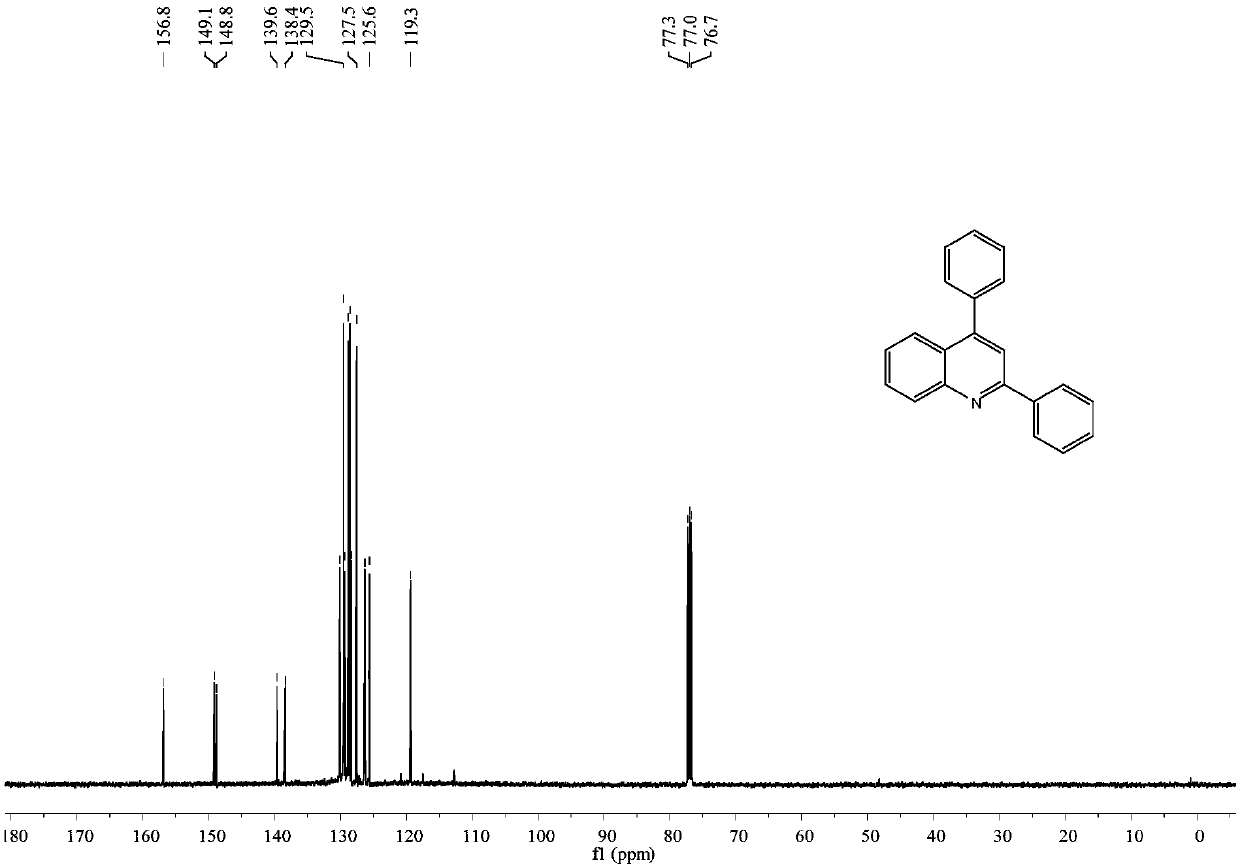
[0036] Embodiment 1: the synthesis of 2,4-diphenylquinoline
[0037] In a 25mL reactor, add N-benzylaniline (0.037g, 0.2mmol), phenylacetylene (0.102g, 1.0mmol), add trifluoroacetic acid (0.003g, 0.03mmol) under stirring, 80 ℃, oxygen bulb conditions Under stirring for 24h. Column chromatography separation (silica gel, 200-300 mesh; developer, petroleum ether / dichloromethane=4 / 1) gave 0.047 g of 2,4-diphenylquinoline with a yield of 83%.
[0038] 2,4-Diphenylquinoline
[0039] yellow oily liquid; 1 H NMR (400MHz, CDCl 3 ):δ8.23(d,J=8.4Hz,1H),8.19(d,J=8.4Hz,2H),7.91(d,J=8.3Hz,1H),7.82(s,1H),7.75–7.71 (m,1H),7.57–7.46(m,9H); 13 CNMR (125MHz, CDCl 3 ): δ156.8, 149.1, 148.8, 139.6, 138.4, 130.1, 129.5, 129.5, 129.3, 128.8, 128.6, 128.4, 127.5, 126.3, 125.7, 125.6, 119.3.
Embodiment 2
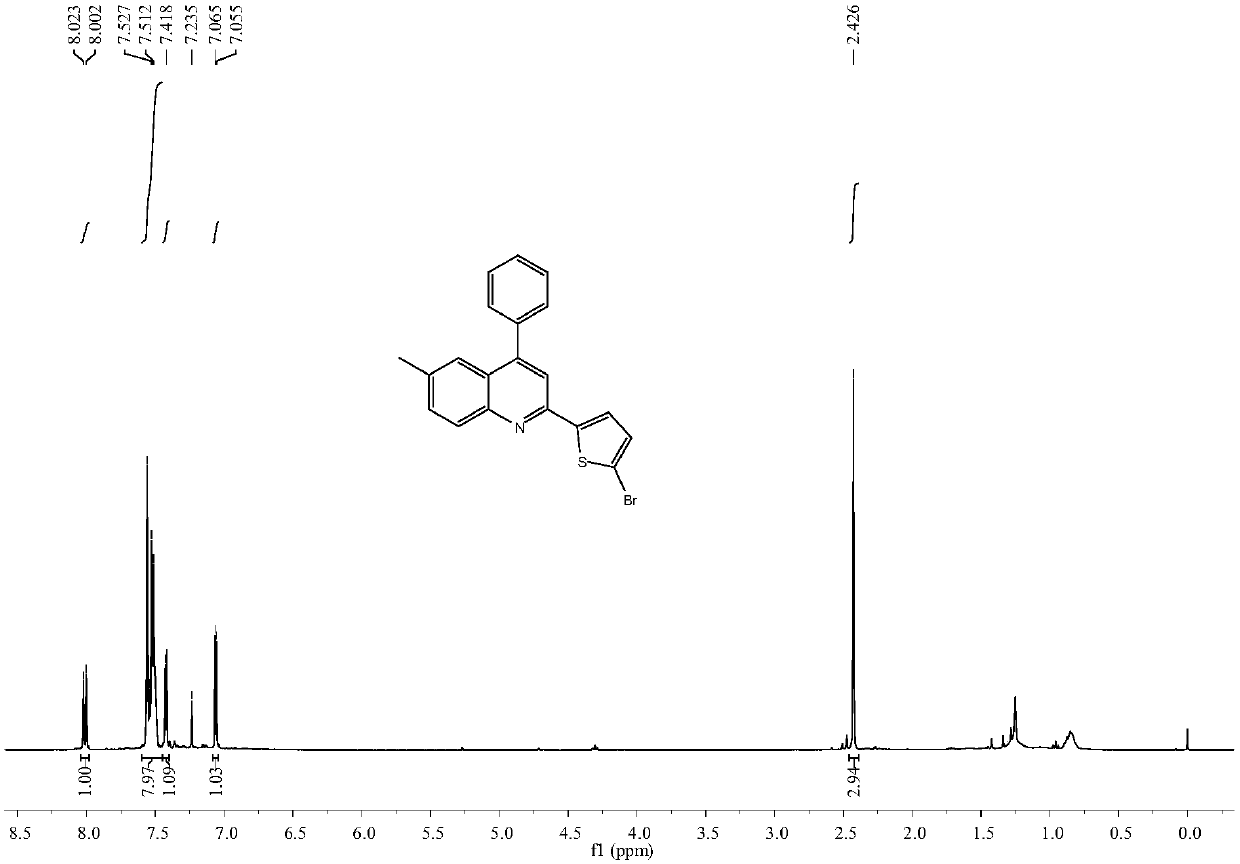
[0040] Embodiment 2: Synthesis of 2-(5-bromothienyl-2-)-6-methyl-4-phenylquinoline
[0041] Operation is the same as in Example 1, by reacting N-[(5-bromothienyl-2-)methyl]-4-methylaniline with phenylacetylene to obtain 2-(5-bromothienyl-2-)-6-methanol Base-4-phenylquinoline 0.059g, yield 78%.
[0042] 2-(5-Bromothienyl-2-)-6-methyl-4-phenylquinoline
[0043] Brown solid; melting point 181.1–182.0°C; 1 H NMR (400MHz, CDCl 3 ):δ8.01(d, J=8.6Hz, 1H), 7.56–7.50(m, 8H), 7.42(d, J=4.0Hz, 1H), 7.06(d, J=4.0Hz, 1H), 2.43 (s, 3H); 13C NMR (100MHz, CDCl 3 ):δ149.8,148.9,146.6,138.0,136.5,132.2,131.0, 129.4,128.9,128.6,128.5,125.8,125.7,124.5,117.1,116.0,21.8; HRMS(ESI,m / z) calcd for C 20 h 15 NBrS + :380.0103[M+H] + ;found:380.0109;IR(neat)3046,2959,2825,1608,1563,1442,1021,850,790,732,574cm -1 .
Embodiment 3
[0044] Embodiment 3: Synthesis of 2-(3,4-dichlorophenyl)-6-methyl-4-phenylquinoline
[0045] The operation is the same as in Example 1, and 2-(3,4-dichlorophenyl)-6-methyl-4- Phenylquinoline 0.067g, yield 92%.
[0046] 2-(3,4-Dichlorophenyl)-6-methyl-4-phenylquinoline
[0047] White solid; melting point 143.0–143.6°C; 1 H NMR (400MHz, CDCl 3 ):δ8.11(d,J=8.5Hz, 1H),7.71–7.68(m,2H),7.62(s,1H),7.58–7.48(m,7H),7.37(dd,J=8.5,2.1 Hz, 1H), 2.47(s, 3H); 13 C NMR (125MHz, CDCl 3 ): δ154.8, 147.5, 147.2, 138.1, 138.1, 137.0, 135.0, 133.1, 132.6, 131.8, 129.8, 129.7, 129.5, 128.5, 128.3, 127.4, 125.6, 124.4, 122.7, 21.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com