Propionylation catalpol derivative and preparation method and application thereof
A technology of derivatives and propionylation, which is applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of restricting the clinical application of catalpol, low fat solubility and low distribution of catalpol, and improve blood Brain barrier permeability, good anti-aging activity, high bioavailability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
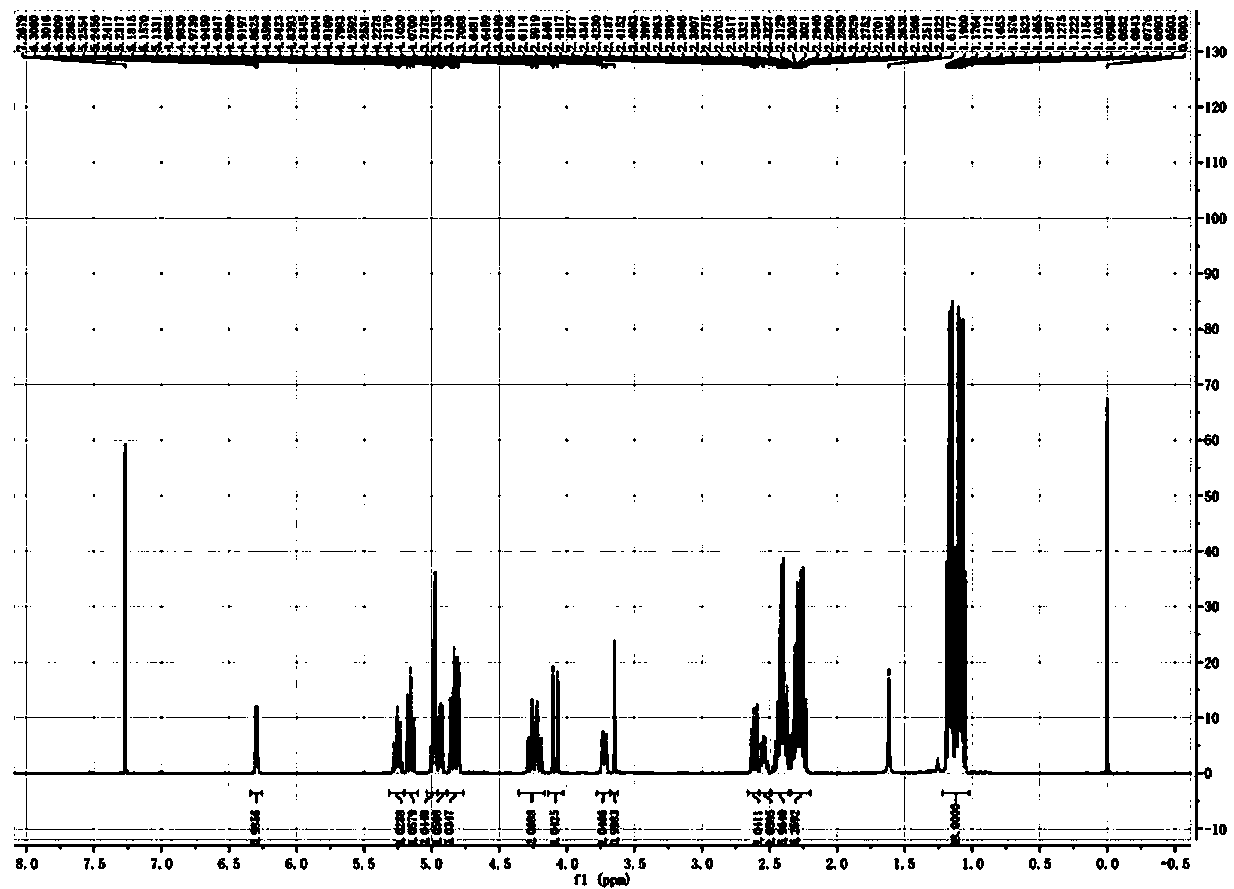
[0046] A propionylated catalpol derivative obtained by esterification of catalpol and propionic anhydride. During esterification, 6 hydroxyl groups or 1 to 5 hydroxyl groups of catalpol participate in the esterification reaction.
[0047] The preparation method of propionylated catalpol derivatives, comprises the following steps:
[0048] 1) Dissolve 100 mg (0.27 mmol) catalpol in 5 mL of pyridine, add 424.57 μL (3.25 mmol) of propionic anhydride, and react at 60° C. for 48 h. The liquid phase monitoring reaction ends, and the mass spectrometry detects that the esterification yield is 96.71 %, evaporate the pyridine to dryness to obtain the product;
[0049] 2) The product of step 1) was dissolved in 40 mL of ethyl acetate, then 40 mL of saturated sodium carbonate solution was added, the layers were allowed to stand, and the lower aqueous phase was discarded (the saturated sodium carbonate solution was added, and the layers were left to stand, and the lower aqueous phase was ...
Embodiment 2
[0053] A propionylated catalpol derivative obtained by esterification of catalpol and propionic anhydride. During esterification, part of the hydroxyl groups of catalpol participates in the esterification reaction.
[0054] The preparation method of propionylated catalpol derivatives, comprises the following steps:
[0055] 1) Dissolve 0.27 mmol catalpol in 6.5 mL pyridine, add 1.63 mmol propionic anhydride, react at 30° C. for 24 h, and evaporate pyridine to dryness to obtain the product;
[0056] 2) Dissolve the product of step 1) in 30 mL of ethyl acetate, add 20 mL of saturated sodium carbonate solution, leave to stand for stratification, discard the lower aqueous phase (add saturated sodium carbonate solution, leave to stand for stratification, discard the lower aqueous phase, this The steps were repeated 3 times), the organic phase was dried to remove water using anhydrous sodium sulfate, evaporated to dryness ethyl acetate, column chromatography, evaporated to dryness ...
Embodiment 3
[0059] A propionylated catalpol derivative obtained by esterification of catalpol and propionic anhydride. During esterification, part of the hydroxyl groups of catalpol participates in the esterification reaction.
[0060] The preparation method of propionylated catalpol derivatives, comprises the following steps:
[0061] 1) Dissolve 0.27 mmol catalpol in 4 mL pyridine, add 2.43 mmol propionic anhydride, react at 90°C for 12 h, evaporate the pyridine to dryness to obtain the product;
[0062] 2) Dissolve the product of step 1) in 40 mL of ethyl acetate, add 40 mL of saturated sodium carbonate solution, leave to stand for stratification, discard the lower aqueous phase (add saturated sodium carbonate solution, leave to stand for stratification, discard the lower aqueous phase, this The steps were repeated 3 times), the organic phase was dried to remove water using anhydrous sodium sulfate, evaporated to dryness ethyl acetate, column chromatography, evaporated to dryness of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com