Application of penylpropanoids Leucosceptoside A to preparation of anticomplement medicines
A technology for phenylpropanoid glycosides and compounds, which is applied in the field of traditional Chinese medicine pharmacy and can solve the problems such as the complement inhibitory activity of no phenylpropanoid glycoside compound, anthoside A, and the like.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Preparation of Phenylpropanoid Glycoside A
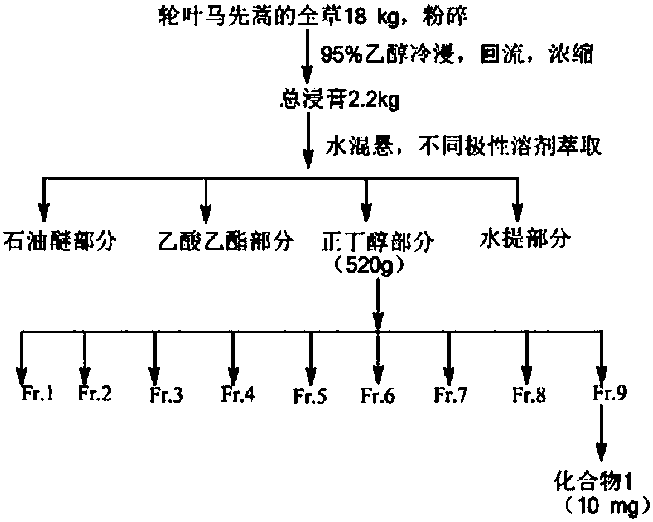
[0022] Take 18 kg of dried Artemisia versicolor whole herb, crush it, soak it in 95% ethanol (60 L) at room temperature for 3 times, combine the extracts and concentrate until there is no alcohol smell to obtain a total extract of 2.2 kg, add water (3500 mL) to the extract Suspended, extracted three times with equal volumes of petroleum ether (60-90°C), ethyl acetate and n-butanol in sequence to obtain petroleum ether fraction (52 g), ethyl acetate fraction (172 g) and n-butanol fraction (520 g ). The n-butanol extraction part was separated by silica gel column chromatography, followed by chloroform-methanol (chloroform, 50:1, 30:1, 15:1, 9:1, 7:1, 5:1, 4:1, 3:1 , 2:1, 1:1) gradient elution, and 9 fractions were obtained. Wherein the fraction Fr.9 is separated by silica gel column chromatography (chloroform-methanol=3:1), MCI column chromatography (methanol-water=50:50) and reverse ODS (methanol-water=50:50) to ob...
Embodiment 2
[0023] Example 2 Anti-complement classical pathway test in vitro
[0024]Take 0.1ml of complement (guinea pig serum), add barbiturate buffer solution (BBS) to prepare a 1:5 solution, and double-dilute with BBS to 1:10, 1:20, 1:40, 1:80, 1: 160, 1:320 and 1:640 solutions; take 1:1000 hemolysin, each concentration of complement and 2% sheep red blood cell (SRBC) each 0.1ml dissolved in 0.3ml BBS, mix well, 37 ℃ water bath for 30min, then put into low temperature Centrifuge in a high-speed centrifuge at 5000rpm and 4°C for 10min, take 0.2ml of the supernatant from each tube and put it in a 96-well plate, measure its absorbance at 405nm, and set up a full hemolysis group (0.1ml of 12% SRBC dissolved in 0.5ml of three Distilled water), using the absorbance of three-distilled water lysed blood vessels as the standard of full hemolysis, calculate the hemolysis rate, take the dilution of complement as the X-axis, and the percentage of hemolysis caused by each dilution of complement as...
Embodiment 3
[0025] Example 3 Anti-complement alternative pathway test in vitro
[0026] Take 0.2ml of complement (human serum), add AP diluent (barbital buffer, pH 7.4, containing 5 mMg 2+ , 8 mMEGTA) was prepared into a 1:5 solution, and double-diluted into 1:10, 1:20, 1:40, 1:80, 1:160, 1:320 and 1:640 solutions, and each concentration was taken Complement 0.15ml, AP diluent 0.15ml and 0.5% rabbit erythrocyte (RE) 0.20ml, mix well, put in a low-temperature high-speed centrifuge at 37°C for 30 minutes, centrifuge at 5000 rpm and 4°C for 10 minutes, take Put 0.2 ml of the supernatant in each tube into a 96-well plate, and measure the absorbance at 405 nm. At the same time, a complete hemolysis group (0.20 ml 0.5% RE dissolved in 0.3 ml triple-distilled water) was set up in the experiment. The absorbance of triple-distilled water-lyzed blood vessels was used as the standard for full hemolysis. Hemolysis rate, take the dilution of complement as the X-axis, and the percentage of hemolysis c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com