Vonoprazan salts and crystal forms, and preparation method and application thereof
A technology of crystal form and salt crystal, applied in Vonorazan salt, preparation of anti-gastric acid secretion drugs, crystal form and its preparation, which can solve the problems of difficulty in sterility assurance and high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
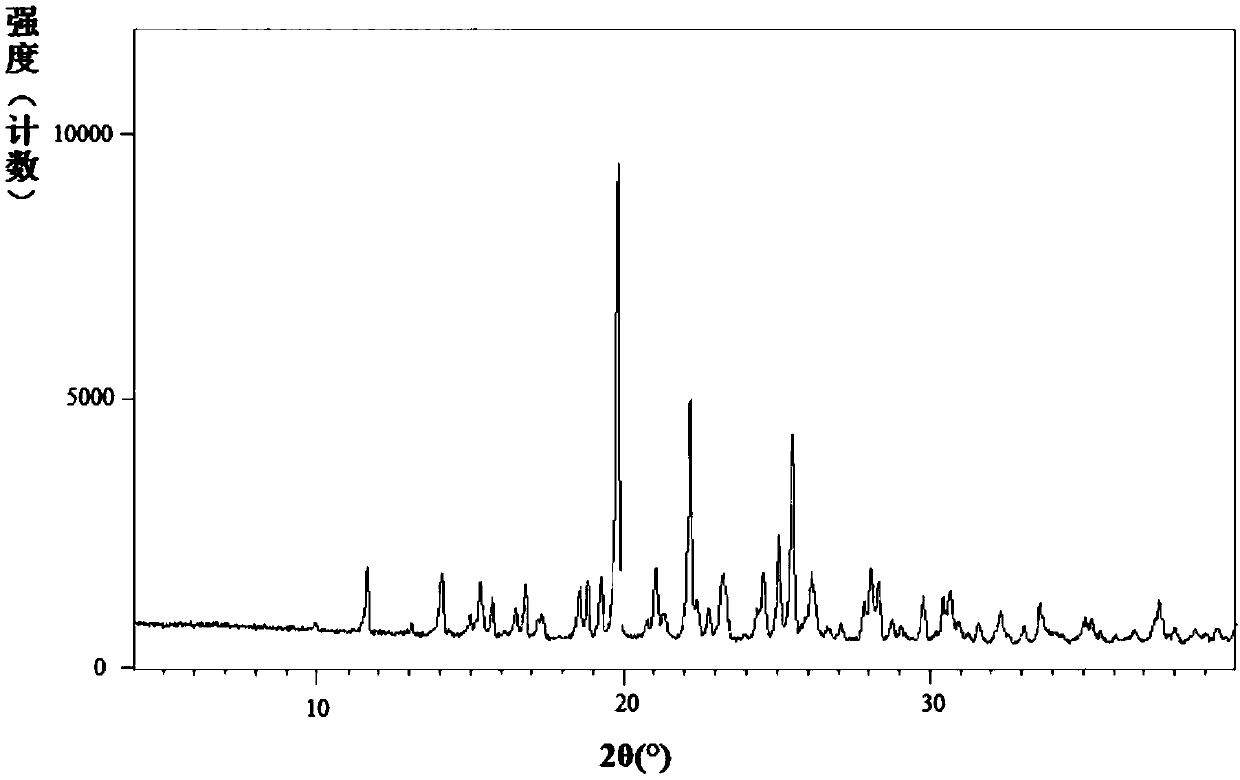
Image
Examples
Embodiment 1
[0366] Example 1: Preparation of Vonorazan Dihydrochloride (Formula III) and Form A thereof
[0367] Dissolve 3.00 g (8.69 mmol) of Vonorazan in 6 ml of ethanol, and 1.59 g (15.64 mmol, 1.8 eq) of concentrated hydrochloric acid (containing 36% hydrogen chloride) in 3 ml of ethanol. At 40-45°C, the hydrochloric acid ethanol solution was added dropwise to the Vonorazan ethanol solution, and then 20 ml of ethyl acetate was added dropwise. Cool to about 5°C. Filtration and washing with ethyl acetate gave Vonorazan dihydrochloride.
[0368] The content of vonorazan was determined by HPLC to be consistent with vonorazan dihydrochloride.
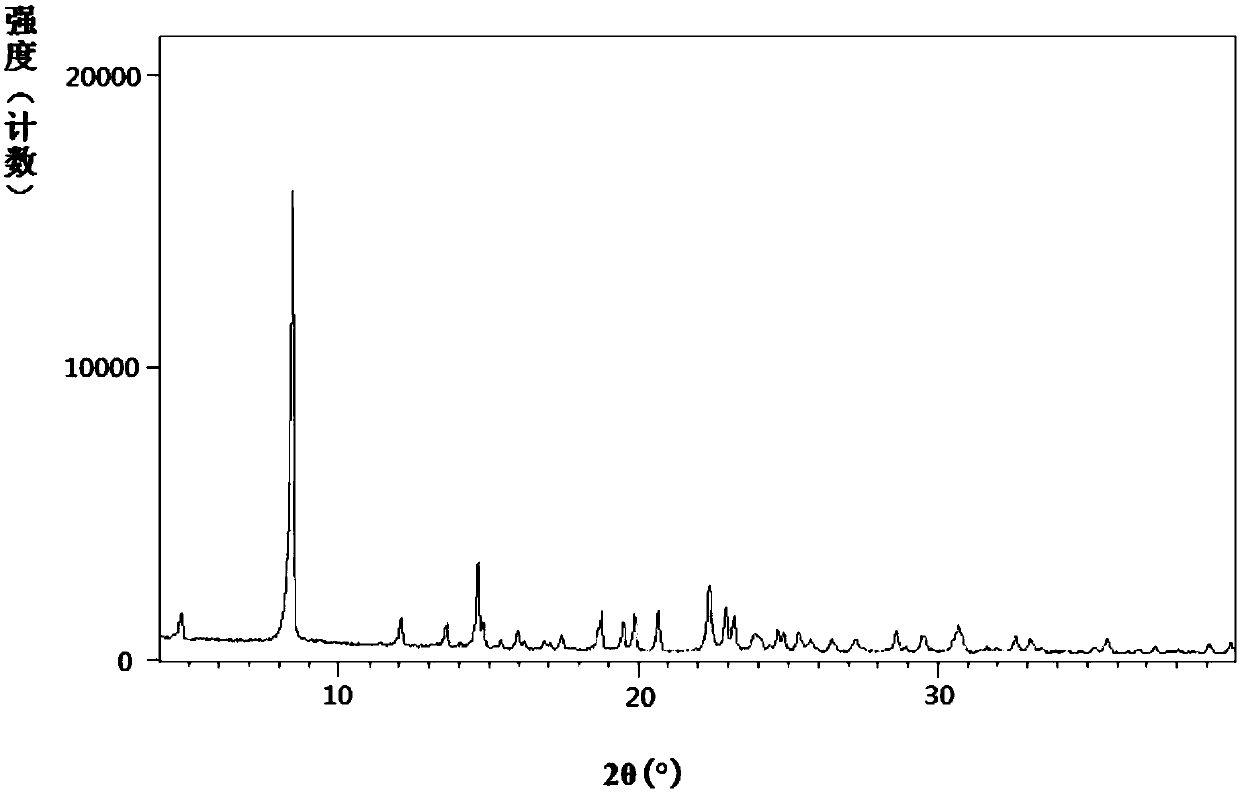
[0369] The powder X-ray diffraction pattern of measured Vonorazan dihydrochloride is shown in figure 1 , the measured value is as follows (take the measured value corresponding to the diffraction peak with a relative intensity greater than 2% within the range of 2θ angle 4°-40°, the measured values of 2θ and d are rounded to three decimal pla...
Embodiment 2
[0374] Example 2: Preparation of Vonorazan Dihydrochloride (Formula III) and Form B thereof
[0375] Dissolve 3.00 g (8.69 mmol) of Vonorazan in 6 ml of ethanol, and 1.59 g (15.64 mmol, 1.8 eq) of concentrated hydrochloric acid (containing 36% hydrogen chloride) in 3 ml of ethanol. At 40-45°C, add the above hydrochloric acid ethanol solution dropwise to the Vonorazan ethanol solution, and then slowly add 20 ml of ethyl acetate dropwise. Cool to about 5°C. Filtrate, wash with ethyl acetate, and dry under reduced pressure at 40-45°C to obtain Divornorazan dihydrochloride.
[0376] 1 H NMR (400MHz, DMSO-d 6 )δ: 2.477-2.517(m, 4.3H, with DMSO-d 6 Solvent peak overlap), 3.972-4.000(t,2H), 5.393(br.,2H), 6.634-6.639(d,1H), 7.072-7.114(m,1H), 7.212-7.258(m,2H),7.510 -7.568(m,1H),7.617-7.651(m,1H),7.875-7.879(m,1H),7.914-7.945(m,1H),8.571-8.576(d,1H),8.888-8.904(dd, 1H),9.446-9.456(br.,2H).
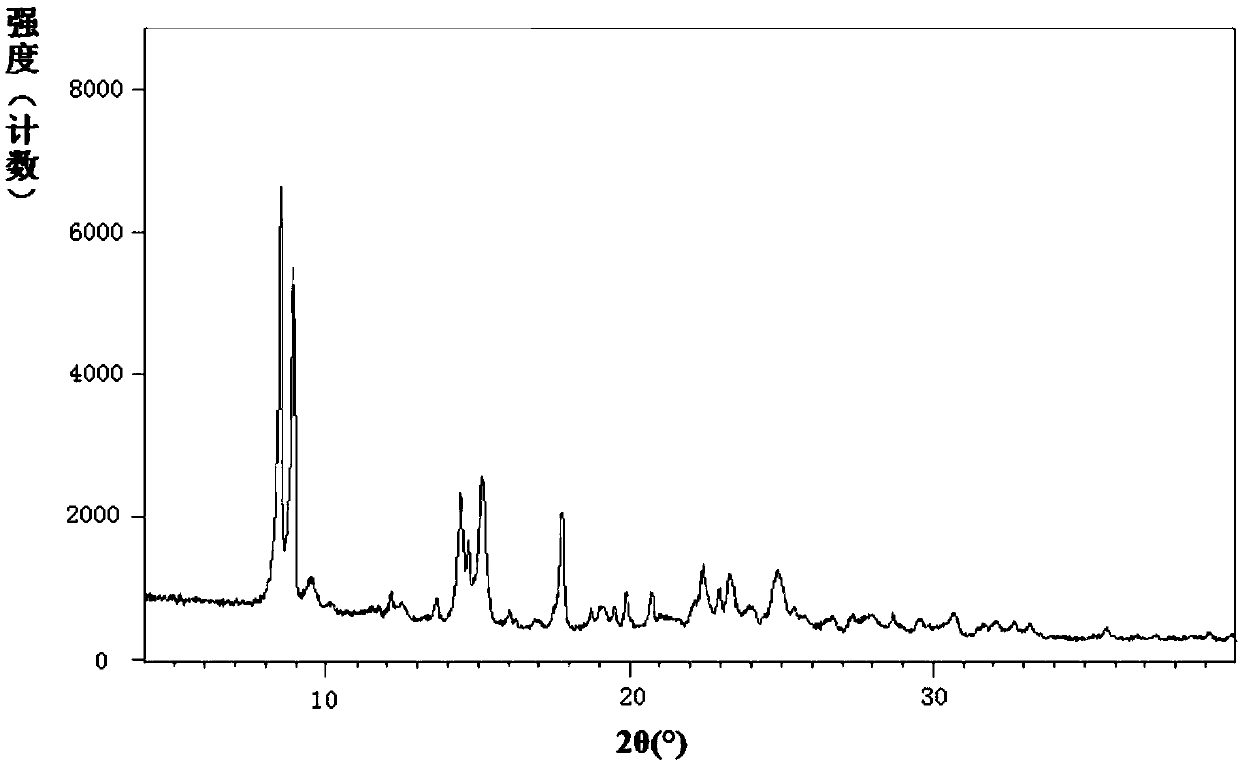
[0377] The content of vonorazan was determined by HPLC to be consistent with vonorazan...
Embodiment 3
[0383] Embodiment 3: Preparation of Vonorazan Dihydrobromide (Formula IV) and Form A thereof
[0384] Dissolve 3.00 g (8.69 mmol) of Vonorazan in 6 ml of ethanol, and 2.78 g (16.51 mmol, 1.9 eq) of hydrobromic acid (containing 48% hydrogen bromide) in 3 ml of ethanol. At 45-50° C., the hydrobromic acid ethanol solution was added dropwise to the Vonorazan ethanol solution, and then 20 ml of acetone was added dropwise. Cool to about 10°C. Filter, wash with acetone, and dry under reduced pressure at 45-50°C to obtain Dihydrobromide of Vonorazan.
[0385] 1 H NMR (400MHz, DMSO-d 6 )δ:2.540-2.567(t,3H),4.020-4.049(t,2H),4.744(br.,2H),6.551-6.555(d,1H),7.087-7.128(m,1H),7.220-7.261 (m,2H),7.519-7.558(m,1H),7.628-7.663(m,1H),7.844-7.849(m,1H),7.886-7.917(m,1H),8.579-8.584(d,1H) ,8.799(br.,2H),8.899-8.915(dd,1H).
[0386] The content of vonorazan was determined by HPLC to be consistent with vonorazan dihydrobromide.
[0387] The powder X-ray diffraction pattern of measured Vono...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com