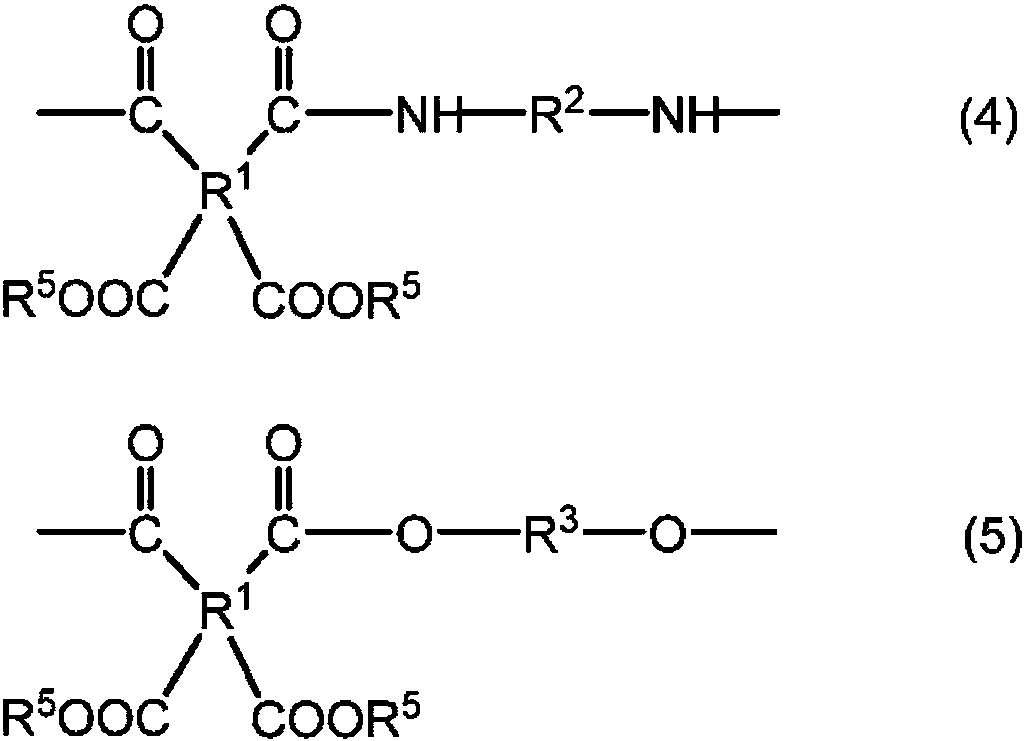
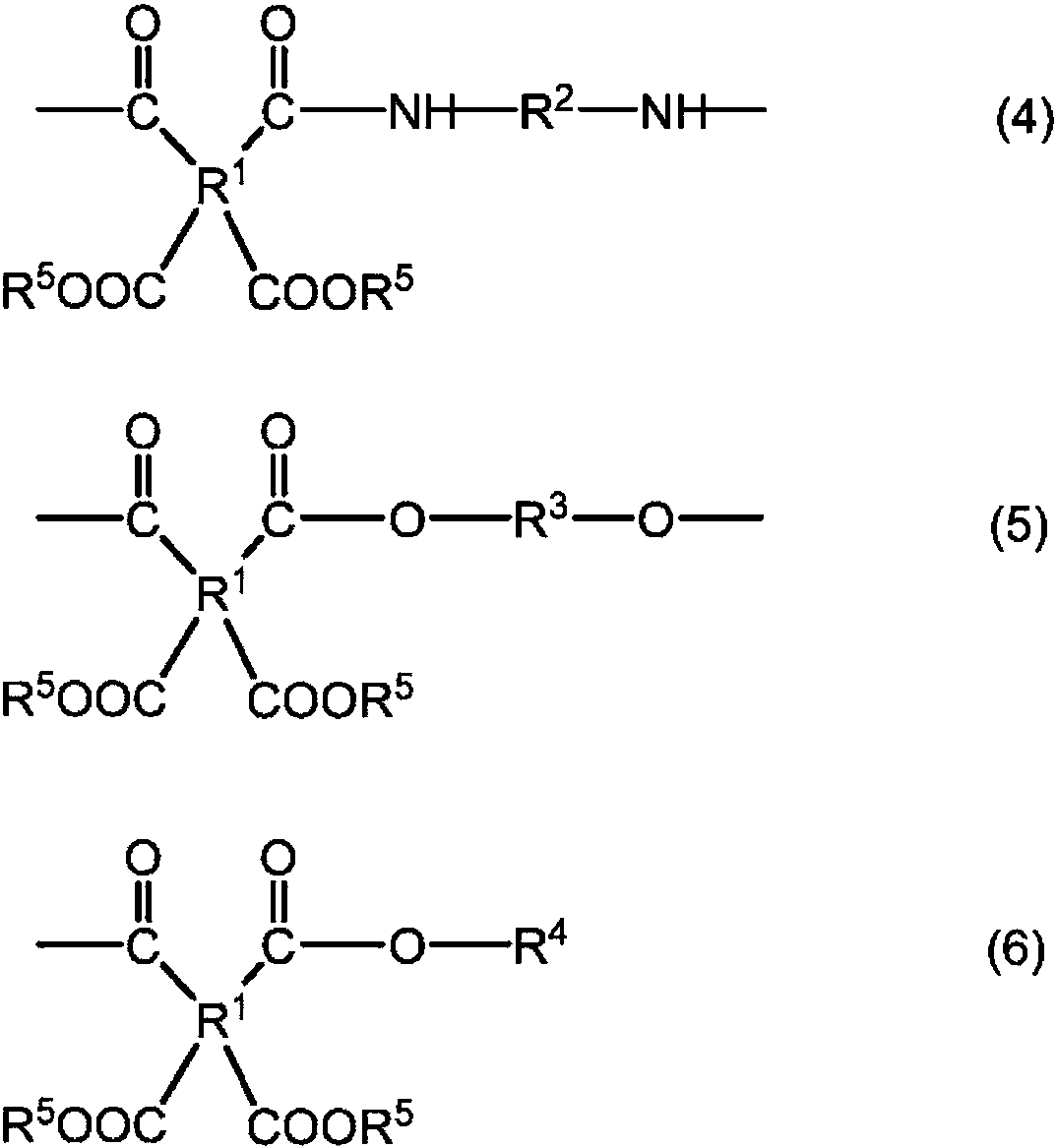
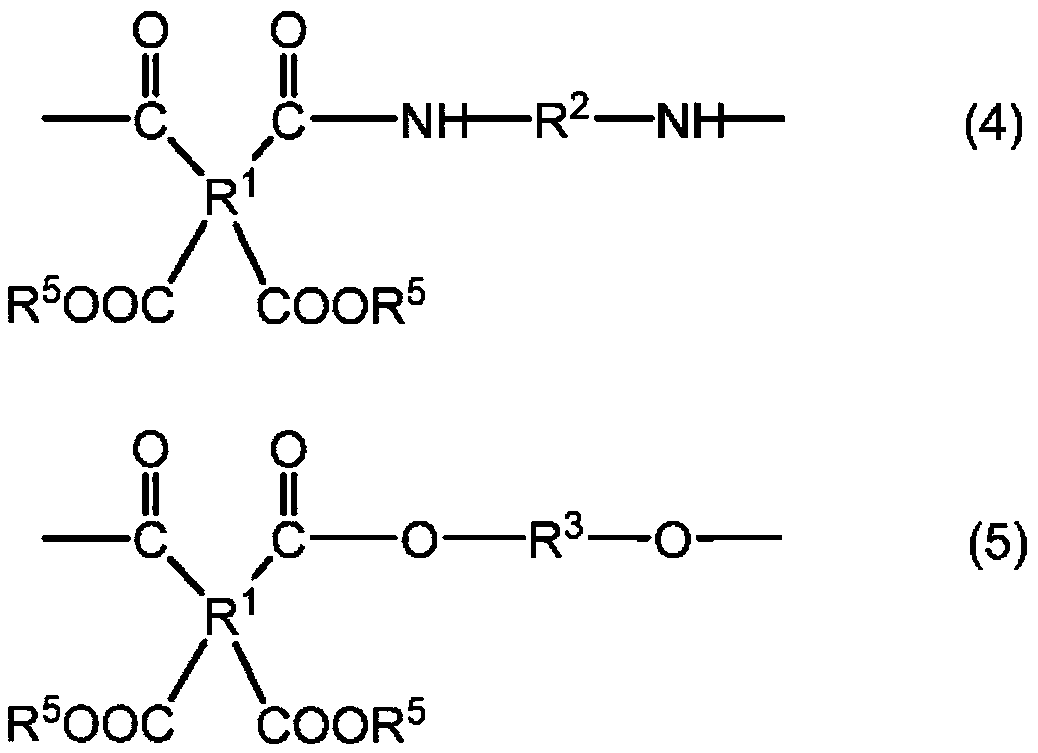
Polyesteramide acids and photosensitive compositions containing same
A polyester amic acid and compound technology, which is applied in the production field of the photosensitive polymer, can solve the problem of unrecorded analytical size, etc., and achieve the effects of excellent residual film rate, excellent analytical performance, and excellent flatness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0245] Next, the present invention will be specifically explained through examples and comparative examples, but the present invention is not limited by these examples at all.
Synthetic example 1
[0246] [Synthesis example 1] Synthesis of polyester amic acid (A1) with polymerizable double bond
[0247] In a four-necked flask equipped with a stirrer, propylene glycol monomethyl ether acetate (PGMEA), 1,2,3,4-butane tetrahydrofuran (PGMEA), 1,2,3,4-butane, which have been dehydrated and purified, are sequentially charged with the following weights: Carboxylic dianhydride (BT-100), SMA1000P (trade name; styrene-maleic anhydride copolymer, Sichuan Petrochemical Co., Ltd.), benzyl alcohol, 4-hydroxybutyl acrylate glycidyl ether (4HBAGE), Stirring was performed at 125°C for 6 hours under a stream of dry nitrogen (the first step of synthesis).
[0248]
[0249] After that, the reacted solution was cooled to 25°C, 3,3'-diaminodiphenylsulfone (DDS) and PGMEA were added with the following weights, and stirred at 20°C to 30°C for 2 hours, and then heated at 125°C Under stirring for 1 hour (the second step of synthesis).
[0250] DDS 0.79g
[0251] PGMEA 6.74g
[0252] [Z / Y=2.7, (Y+Z) / X=0...
Synthetic example 8
[0259] [Synthesis example 8] Synthesis of polyester amic acid (A8) with polymerizable double bond
[0260] In a four-necked flask with a stirrer, propylene glycol monomethyl ether acetate (PGMEA), 1,2,3,4-butane tetracarboxylic dianhydride (BT -100), SMA1000P (trade name; styrene·maleic anhydride copolymer, Sichuan Crude Oil & Chemical Co., Ltd.), benzyl alcohol, stirred for 2 hours at 125°C under a stream of dry nitrogen (the first step of synthesis).
[0261]
[0262] After that, the reacted solution was cooled to 25°C, 3,3'-diaminodiphenylsulfone (DDS) and PGMEA were added with the following weights, and stirred at 20°C to 30°C for 2 hours, and then heated at 125°C Under stirring for 1 hour (the second step of synthesis).
[0263] DDS 0.60g
[0264] PGMEA 4.41g
[0265] After that, the reacted solution was cooled to 25°C, and glycidyl methacrylate (GMA), 1-methylimidazole (NMI), and 4-methoxyphenol (MQ) were added with the following weights at 80°C Stirring was carried out for 8 h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com