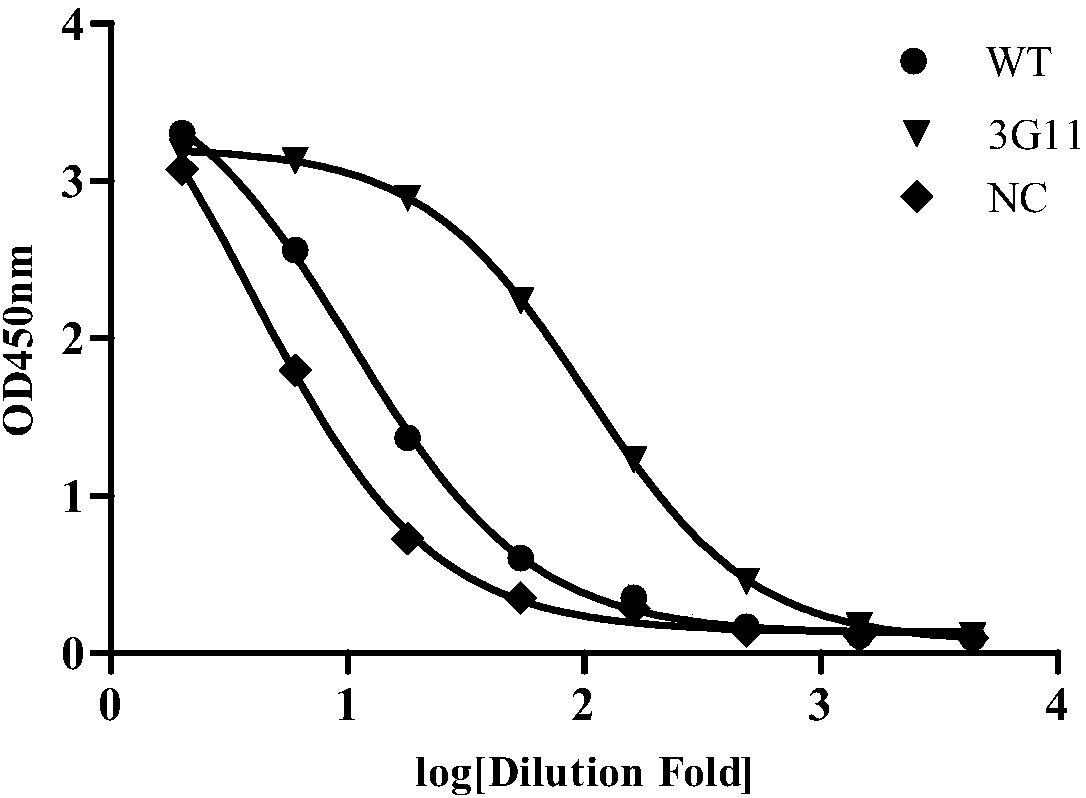
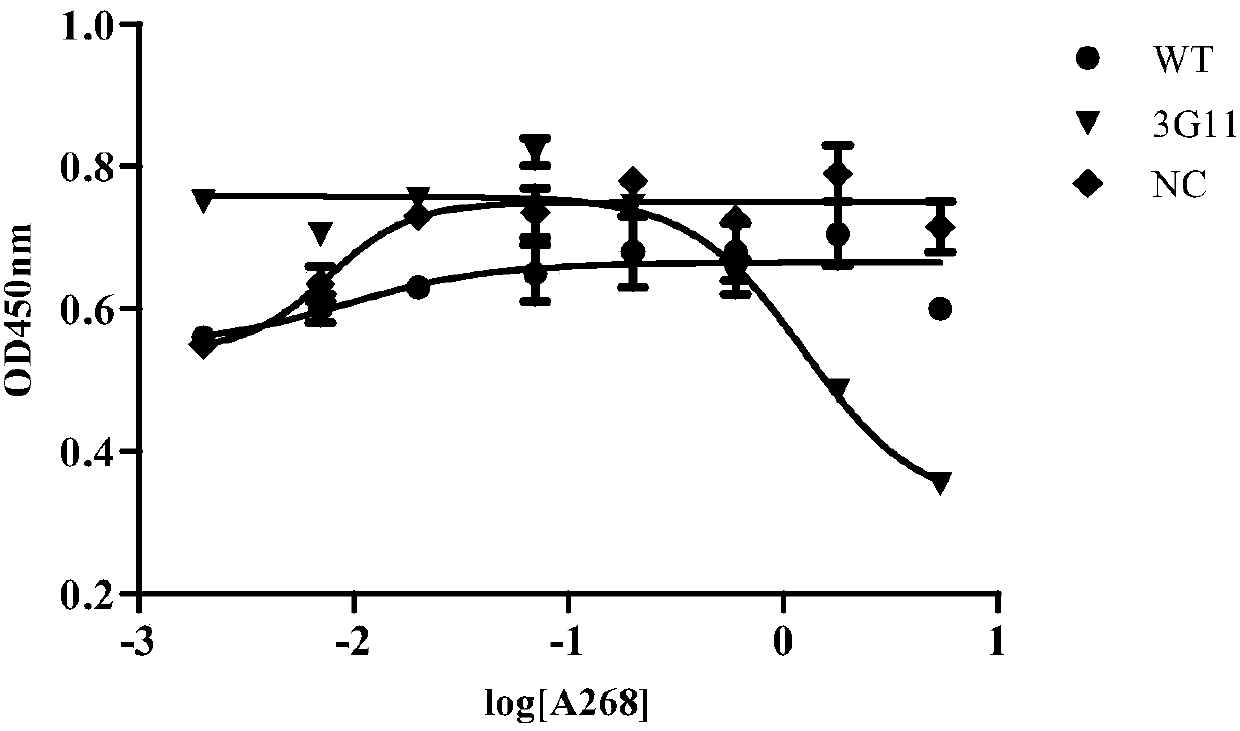
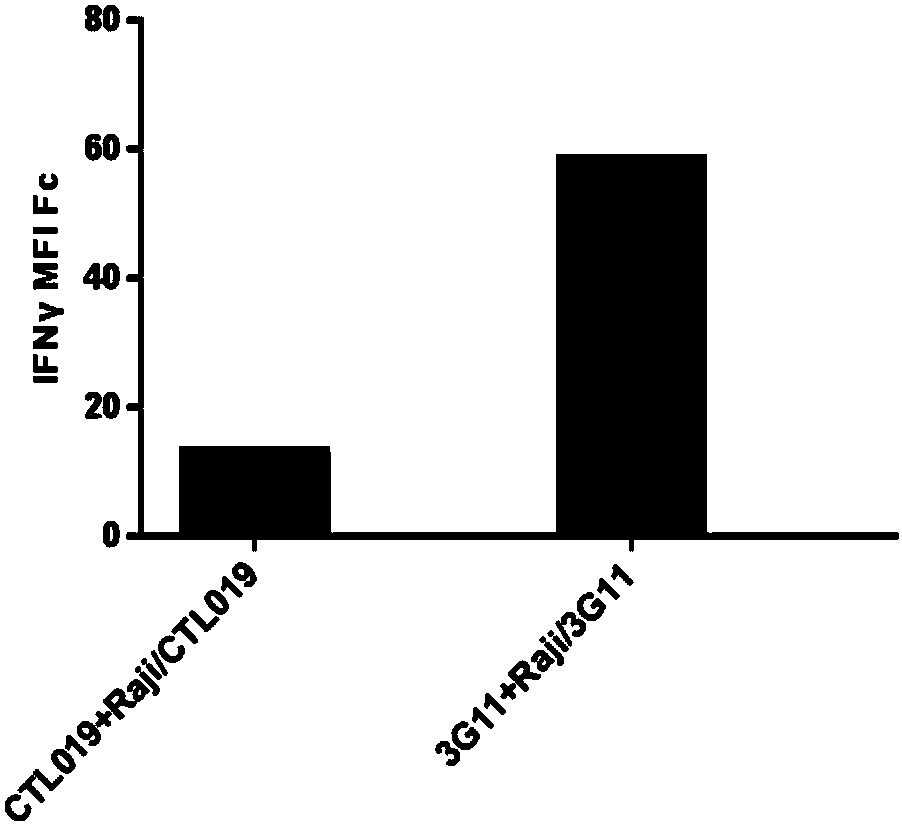
Anti-CD19 antibody and preparation method and use thereof
An antibody and monoclonal antibody technology, applied in the direction of antibody medical components, antibody mimics/stents, anti-tumor drugs, etc., can solve the problems of difficult to obtain curative effects and prolong patient survival.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Construction of the CDR1, CDR2, and CDR3 mutation libraries of the heavy chain (H) and light chain (L) of FMC63scFv:
[0067] The selection of FMC63 heavy chain and light chain CDR regions is based on the amino acid counting method of the variable region (the template is monoclonal antibody FMC63, VH: Y14283.1, VL: Y14284.1), Kabat counting (Kabat number scheme.Bioinf.org. uk) list as follows:
[0068] Table 1
[0069]
[0070]
[0071]Using the pCAN-FMC63scFv plasmid (constructed by inserting the template sequence into the multiple cloning site of the pCANTAB 5E plasmid (purchased from GE), the template sequence is: VH: Y14283.1, VL: Y14284.1) as a template, the mutation was introduced by PCR with random primers , the primers are shown in Table 2. The obtained PCR products of the CDR1, CDR2, and CDR3 mutant libraries of the heavy chain (H) and light chain (L) of FMC63 scFv were named H1, H2, H3, L1, L2, and L3, respectively. After the PCR product and pCANTAB 5...
Embodiment 2
[0075] Panning of phage antibody library:
[0076] Add 20nM CD19-his-biotin antigen and incubate with the phage antibody library at room temperature for 2h, then transfer the mixture to streptavidin magnetic beads and incubate for 15min at room temperature. Unbound phages were washed away with PBST-PBS, and trypsin was added for 30 min at 37°C to elute bound phages. Infect 4ml of TG1 cells in the logarithmic phase with the phages digested and eluted by trypsin, let stand at 37°C for 30 minutes, take part of the bacterial solution to serially dilute for plate counting, and spread the rest of the bacterial solution on 2xYT(GA) plates for next A round of packaging. The packaged phage can be used for the next round of panning. A total of 4 rounds of panning and enrichment were carried out. Each round of panning was 10 times diluted and the antigen concentration was gradually reduced, and the number of PBST-PBS washings was increased round by round (the CD19 -his-biotin antigen c...
Embodiment 3
[0078] Screening and identification of high-affinity scFv:
[0079] After four rounds of panning, single clones were randomly selected, and after IPTG induction, the supernatant was taken for ELISA. After preliminary screening by ELISA, clones with positive signals at least 2 times greater than negative signals were selected and sent for sequencing. clones corresponding to more CDR regions. The concrete steps of described ELISA preliminary screening are as follows:
[0080] Pick colony clones in 4ml 2xYT medium, culture overnight at 37°C 200rpm, transfer to 500ml 2xYT medium at a ratio of 1:100, and culture at 37°C 200rpm until logarithmic growth phase; add M13K07 helper phage (MOI=20), 37 Incubate at ℃ for 30 minutes, then incubate at 37℃ and 200rpm for 30 minutes; remove the supernatant by centrifugation, and resuspend the bacteria in 500ml 2xYT / Kan / Amp resistance medium for overnight culture; use 1 / 5 volume of the overnight culture supernatant The PEG / NaCl solution was pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com