Method for preparing 4-Boc-aminopiperidine
A technology of aminopiperidine and piperidinone, which is applied in the field of preparing 4-Boc-aminopiperidine, can solve the problems of high price of 4-aminopiperidine and difficult industrialized mass production, and avoid the use and operation of metal reducing agents. Simple and shortened production cycle effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
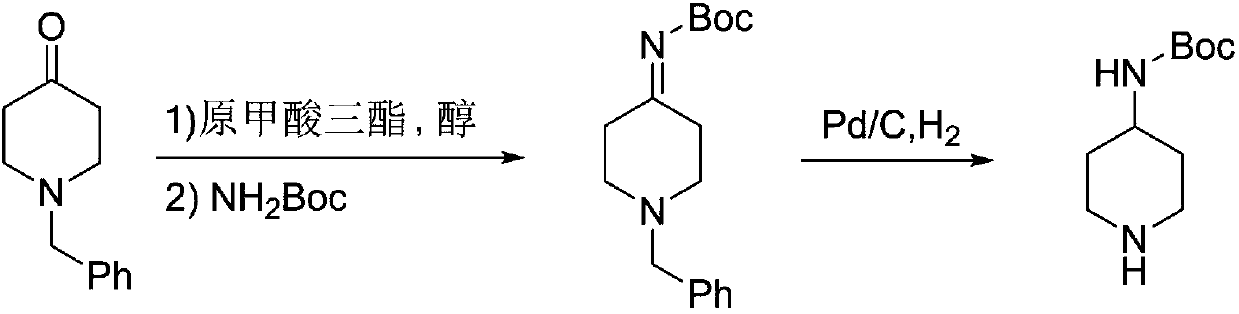
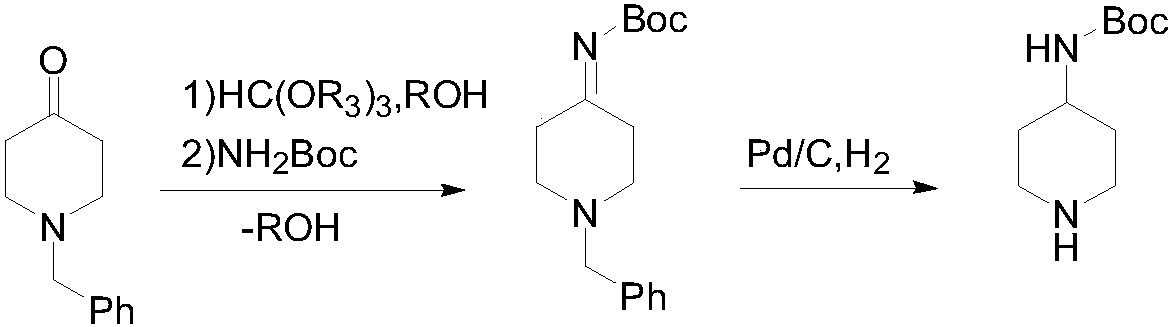
[0025] Step 1: Into a 1L three-necked flask, add 189.2g (1.00mol) of N-benzyl-4-piperidone, 1.72g (10mmol) of p-toluenesulfonic acid, 159.2g (1.50mol) of trimethyl orthoformate and Methanol 400mL, heated to reflux for 2 hours, after the methanol was distilled off at normal pressure, 117.1g (1.00mol) of tert-butyl carbamate (1.00mol) and toluene 600mL were added, heated to 80-100°C, and the methanol produced by the reaction was distilled off while reacting to accelerate the reaction. After reacting for 3 hours, the residual raw material in GC was less than 1%, lowered the temperature, concentrated the solvent under reduced pressure, added ethanol, lowered the temperature to 0°C and stirred for 1 hour, a white solid was precipitated, filtered to obtain 240.5g white solid product, the yield was 83.4%, Purity 99.1%, LCMS (ESI) m / z: [M+H] + 289.18.
[0026] Step 2: Into a 5L autoclave, add 240.5g (0.834mol) of the product obtained in the previous step and 2.4L of methanol, add 24....
Embodiment 2
[0028] Step 1: Add 189.2g (1.00mol) of N-benzyl-4-piperidone, 0.53g (10mmol) of ammonium chloride, 106.1g (1.00mol) of trimethyl orthoformate and methanol into a 1L three-necked flask 400mL, heated to reflux for 2 hours, after distilling off methanol at normal pressure, add 140.6g (1.20mol) of tert-butyl carbamate and 600mL of toluene, heat to 80-100°C, and distill off the methanol generated during the reaction to accelerate the reaction. After 3 hours, the remaining raw material in GC was less than 1%, lowered the temperature, concentrated the solvent under reduced pressure, added ethanol, lowered the temperature to 0°C and stirred for 1 hour, a white solid precipitated, filtered to obtain 235.3g white solid product, the yield was 81.6%, the purity 99.0%.
[0029] Step 2: Add 235.3g (0.816mol) of the product obtained in the previous step and 2.3L of methanol to a 5L autoclave, add 11.8g of 5% Pd / C, replace the system with nitrogen three times, and feed hydrogen to control the...
Embodiment 3
[0031] Step 1: Into a 1L three-necked flask, add 189.2g (1.00mol) of N-benzyl-4-piperidone, 8.60g (50mmol) of p-toluenesulfonic acid, 296.4g (2.00mol) of triethyl orthoformate and 400mL of ethanol, heated to reflux for 2 hours, after distilling off the ethanol at normal pressure, add 128.9g (1.10mol) of tert-butyl carbamate and 600mL of toluene, heat to 90-110°C, and distill off the ethanol generated during the reaction to accelerate the reaction. After reacting for 3 hours, the remaining raw materials were controlled in GC <1%. Cool down, concentrate the solvent under reduced pressure, add ethanol, cool down to 0°C and stir for 1 hour, a white solid precipitated, filtered to obtain 242.0 g of white solid product, yield 83.9%, 99.2% purity.
[0032] Step 2: Add 242.0g (0.839mol) of the product obtained in the previous step and 2.4L of methanol to a 5L autoclave, add 12.1g of 10% Pd / C, replace the system with nitrogen three times, and feed hydrogen to control the pressure at 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com