A kind of bipolar host material and its preparation method and application
A host material and bipolar technology, which is applied in the field of bipolar host materials and preparation, can solve the problems of reduced lifetime, quenching, and reduced luminous efficiency of devices, so as to reduce the degree of conjugation, improve efficiency, and improve singlet energy. level effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
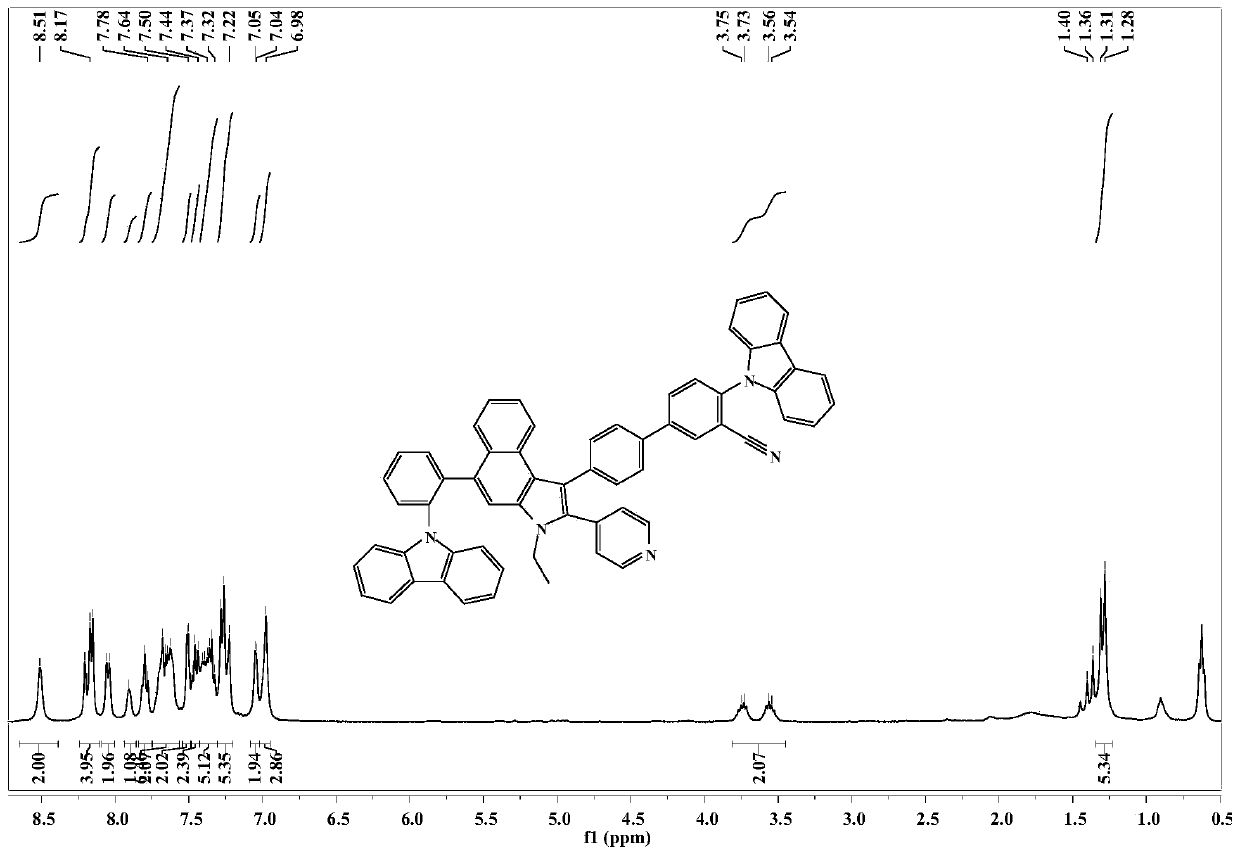
[0044] Embodiment 1: the synthesis of compound CzCNPyCa-1
[0045] The specific implementation method is as follows:
[0046] Step 1, add 2-naphthol (14.71g, 100mmol), p-bromobenzaldehyde (18.5g, 100mmol), 4-(aminomethyl)pyridine (10.81g, 100mmol) into a 250mL reaction flask, pump nitrogen three times , turn on the heating stirrer and raise the temperature to 120°C. After 12 hours of reaction, cool down to 90°C, add 42mL of ethanol for ultrasonication, and filter with suction after cooling to obtain a yellow solid, which is purified by column chromatography (eluent is ethyl acetate:petroleum ether=5: 1) Obtain 30.15 g of light yellow powder (75% yield).
[0047]
[0048] Step 2, add compound a 1-(4-bromophenyl)-2-(pyridin-4-yl)-2,3-dihydro-1H-benzo[e]indole (1.1603g , 2.89mmol), NBS (1.0814g, 6.08mmol), evacuated nitrogen 3 times, added 6mL DMF as a solvent in the dark, turned on the stirrer and stirred overnight. After the reaction, it was extracted with ethyl acetate a...
Embodiment 2
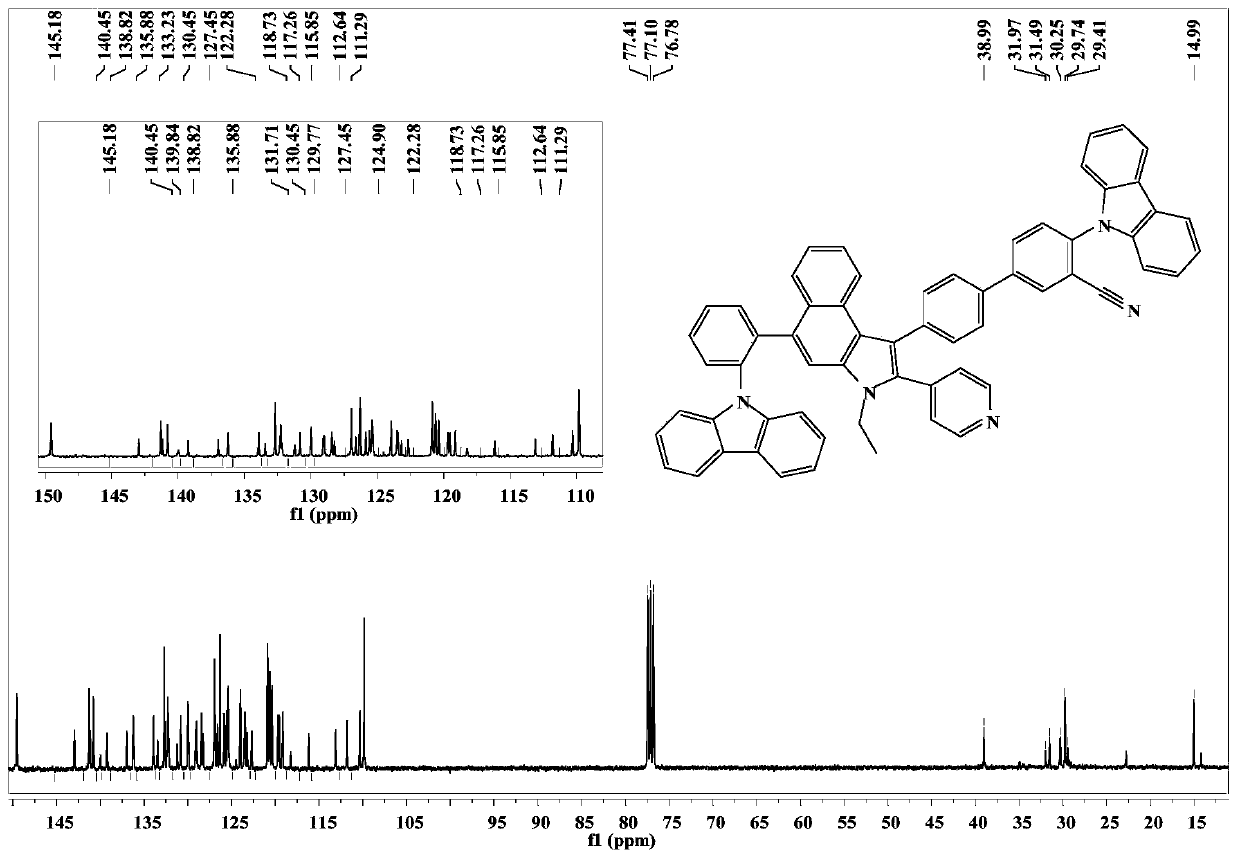
[0061] Embodiment 2: the synthesis of compound CzCNPyCa-2
[0062] The specific implementation method is as follows:
[0063] The synthetic method of compound CzCNPyCa-2 is similar to the synthetic synthetic method of CzCNPyCa-1, and difference is, in embodiment 1 step 6, reactant 9-(2-(4,4,5,5-tetramethyl- 1,3,2-dioxaborolan-2-yl)phenyl)-9H-carbazole was replaced by 3-(9H-carbazol-9-yl)phenylboronic acid, the rest of the operation was the same, and finally yellow Solid powder CzCNPyCa-20.042g (29.8% yield).
[0064]
[0065] 1 H NMR (400MHz, CDCl3) δ8.62(s,11H),8.26(s,3H),8.26–8.17(m,18H),8.13(s,6H),8.20–7.85(m,36H),7.85– 7.65(m,40H),7.61(s,7H),7.59–7.42(m,35H),7.38–7.10(m,55H),4.27(t,J=41.2Hz,13H),1.09–1.04(m, 2H).
[0066] 13 C NMR(101MHz,CDCl3)δ149.72(s,25H),143.58(s,4H),141.29(s,3H),140.80(s,26H),140.12(s,3H),139.34(s,4H) ,137.88(s,5H),136.98(s,4H),136.52(s,3H),135.64(s,4H),133.89(s,3H),132.76(s,38H),132.37(s,27H), 130.04(s,14H),129.79(s,13H),129.35(s,11H),129....
Embodiment 3
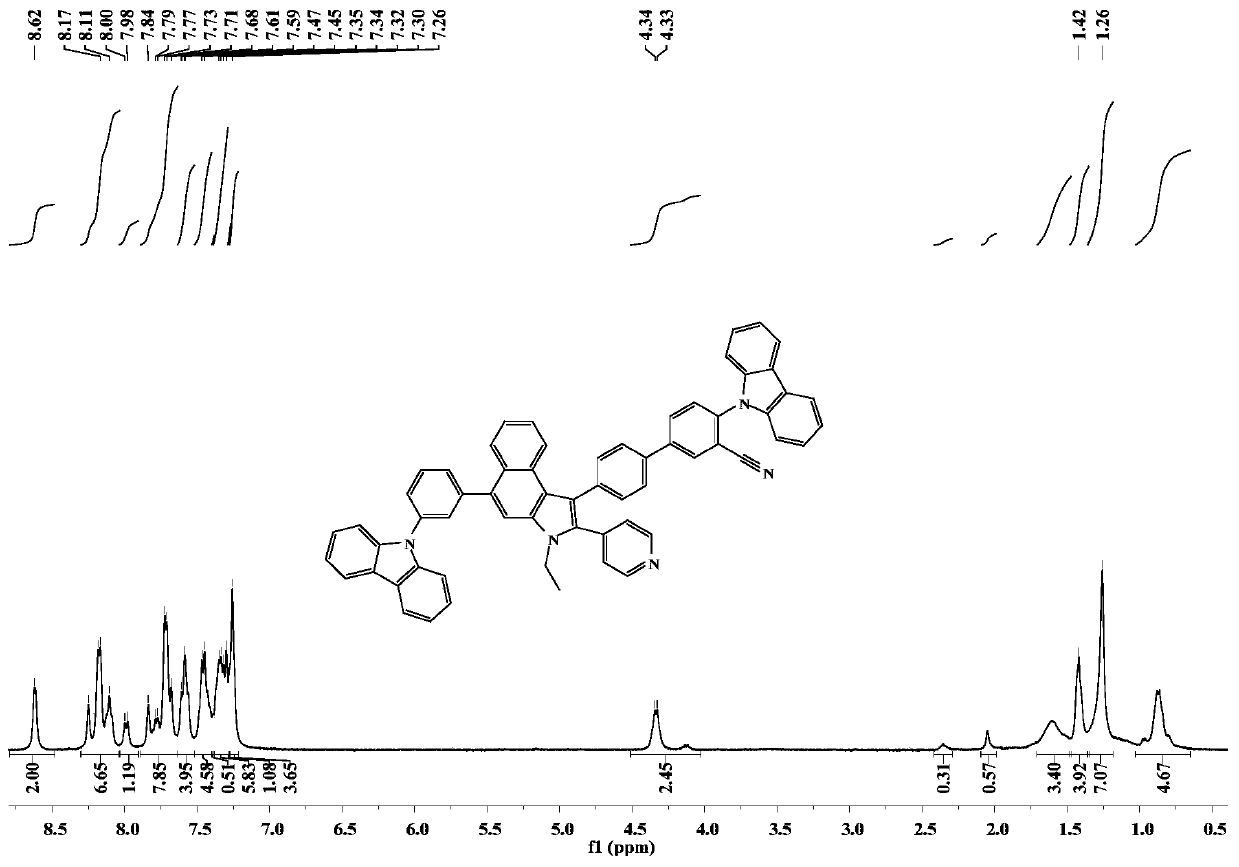
[0067] Embodiment 3: the synthesis of compound CzCNPyCa-3
[0068] The specific implementation method is as follows:
[0069] The synthetic method of compound CzCNPyCa-3 is similar to the synthetic synthetic method of CzCNPyCa-1 in Example 1, and the difference is that in Example 1 step 6, the reactant 9-(2-(4,4,5,5- Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)-9H-carbazole is replaced by 4-(9H-carbazol-9-yl)phenylboronic acid, and the rest are the same , the final yellow solid powder CzCNPyCa-2 0.12 (yield 49.3%).
[0070]
[0071] 1 H NMR (400MHz, DMSO) δ8.63 (dd, J = 14.4, 3.6Hz, 15H), 8.40 (d, J = 8.5Hz, 6H), 8.29 (d, J = 7.7Hz, 19H), 8.10–7.92 (m,21H),7.92–7.75(m,30H),7.59(s,3H),7.59–7.22(m,89H),4.40(d,J=7.0Hz,12H),1.61–0.67(m,49H ).
[0072] 13C NMR(101MHz, CDCl3)δ149.87(s,21H),141.30(s,5H),140.86(d,J=13.2Hz,25H),140.04(s,4H),139.34(s,5H),136.95 (d,J=16.6Hz,10H),136.51(s,4H),135.85(s,4H),133.90(s,4H),132.78(d,J=7.5Hz,28H),132.39(s,20H) ,131.82(s,18H),130.05(s,1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com