N-type organic interface material and preparation method and application thereof
An interface material and organic technology, applied in organic chemistry, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., can solve problems such as insufficient electron transport capacity and poor solubility, achieve excellent thermal stability, and improve electron transport performance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0041] Preparation of Material IX Compound:
[0042]
[0043] 1-Bromo-N,N'-bis(undecyl)perylene-3,4,9,10-tetracarboxylic acid diimide (1.15g, 1.5mmol) and 4-(3-(1-imidazole Base) propylaminomethyl) phenylboronic acid pinacol ester (1.53g, 4.5mmol) and TBAB (0.28g, 0.86mmol) were put into a 100mL two-necked reaction flask, the nitrogen gas was replaced three times, and then added to the reaction flask Pd(PPh 3 ) 4 (0.5g, 0.43mmol) and it was sealed, and the nitrogen gas was changed three times. Inject 45 mL of deoxygenated toluene and 2 mol / L of K into the reaction flask 2 CO 3 15 mL of the solution was reacted at 95°C for 24h. After the reaction was completed, the reaction was quenched with water, cooled to room temperature, extracted with dichloromethane, suction filtered, column chromatography, and dried to obtain the target product material IX (0.36 g, yield 21.4%).
Embodiment 1-2
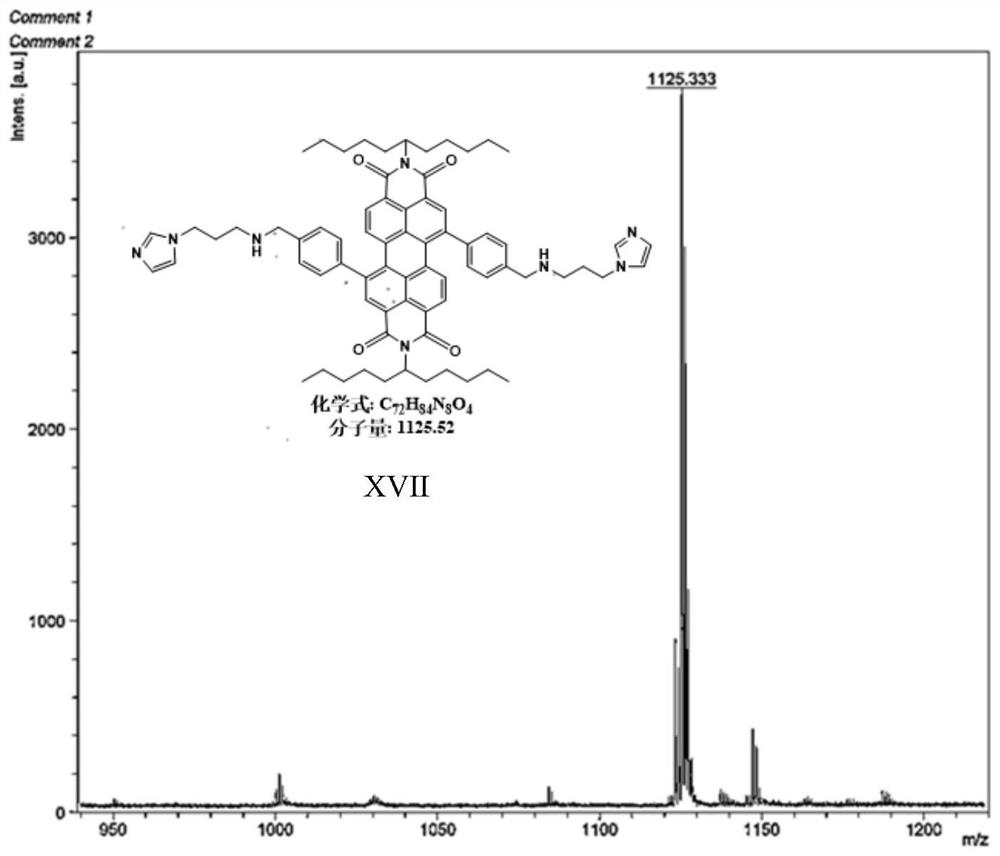
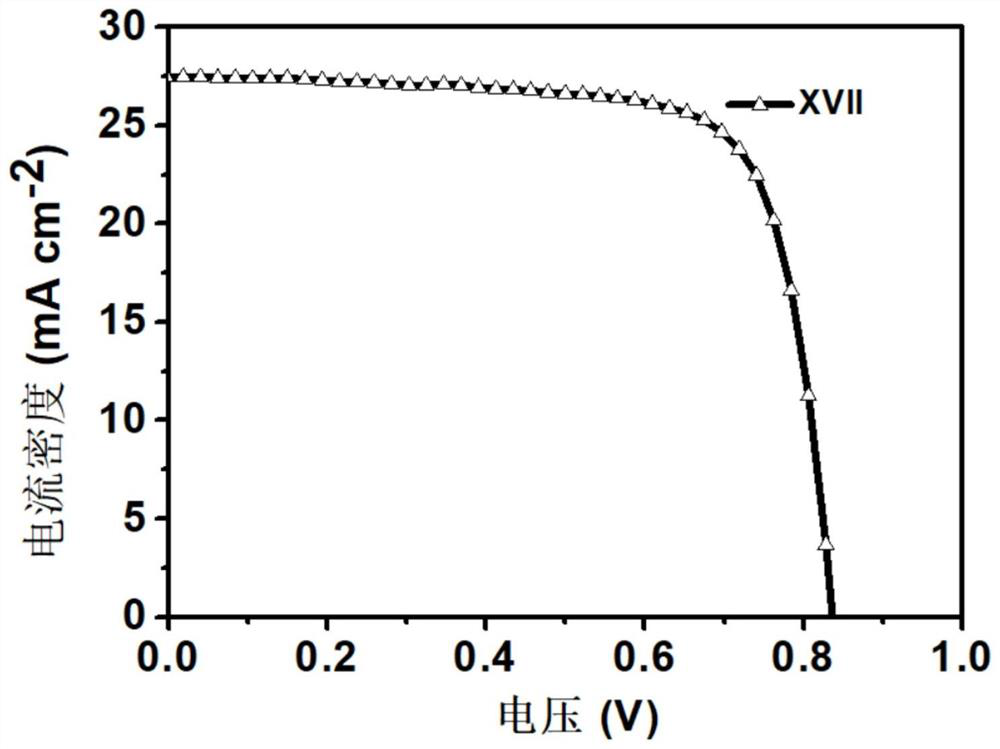
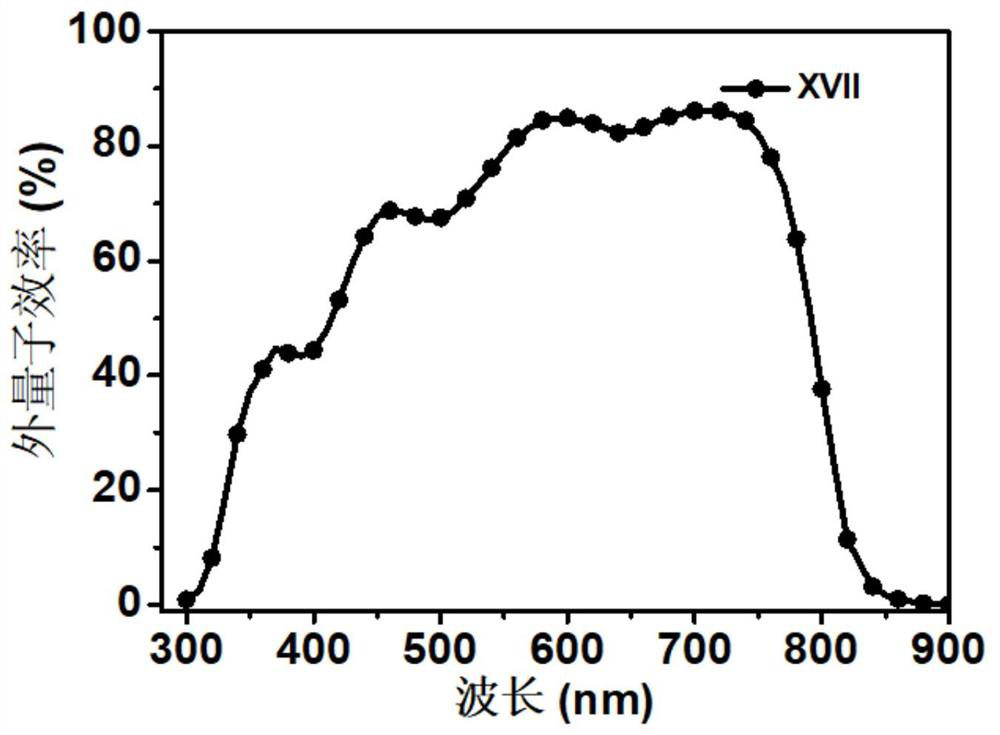
[0045] Preparation of Material XVII Compounds:
[0046]
[0047] 1,7-dibromo-N,N'-bis(undecyl)perylene-3,4,9,10-tetracarboxylic acid diimide (0.9g, 1mmol), 4-(3-(1 -imidazolyl) propylaminomethyl) phenylboronic acid pinacol ester (1.3g, 12mmol), tetrabutylammonium bromide (TBAB) (0.27g, 0.8mmol) are put into the two-necked bottle of 150mL, it is used Rubber stopper seal, replacement N 2 three times. Then quickly add Pd(PPh 3 ) 4 Catalyst (0.49mg, 0.43mmol), and then replace N 2three times. Finally, bubbled deoxygenated toluene (40 mL) and 2M K 2 CO 3 Aqueous solutions (15 mL) were injected into reaction flasks respectively, and reacted at 95° C. for 24 h. After the reaction, extract 3 times with DCM solvent and saturated NaCl solution, the separated organic layer was washed with MgSO 4 Suction filtration after drying and the crude product obtained after concentrating the solution were separated and purified by column chromatography (eluent PE:DCM=4:1), and dried in ...
Embodiment 1-3
[0049] Preparation of Material XXV Compounds:
[0050]
[0051] 1,5,7-tribromo-N,N'-bis(undecyl)perylene-3,4,9,10-tetracarboxylic acid diimide (0.47g, 0.5mmol) and 4-(3 -(1-imidazolyl) propylaminomethyl) phenylboronic acid pinacol ester (0.51g, 1.5mmol) and TBAB (0.09g, 0.28mmol) were put into a 100mL two-necked reaction flask, and the nitrogen gas was changed three times, and then Add Pd(PPh 3 ) 4 (0.5g, 0.43mmol) and it was sealed, and the nitrogen gas was changed three times. Inject 45 mL of deoxygenated toluene and 2 mol / L of K into the reaction flask 2 CO 3 15 mL of the solution was reacted at 95°C for 24 hours. After the reaction was completed, the reaction was quenched with water, cooled to room temperature, extracted with dichloromethane, suction filtered, column chromatography, and dried to obtain the target product material XXV (0.45 g, yield 33.4%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com