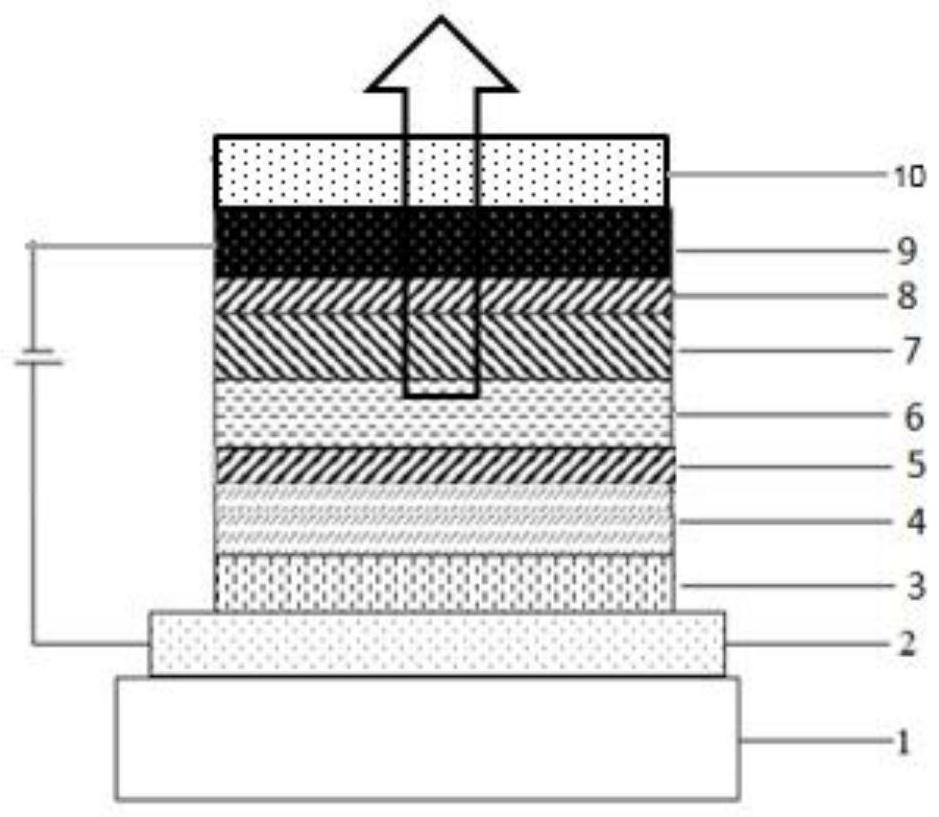
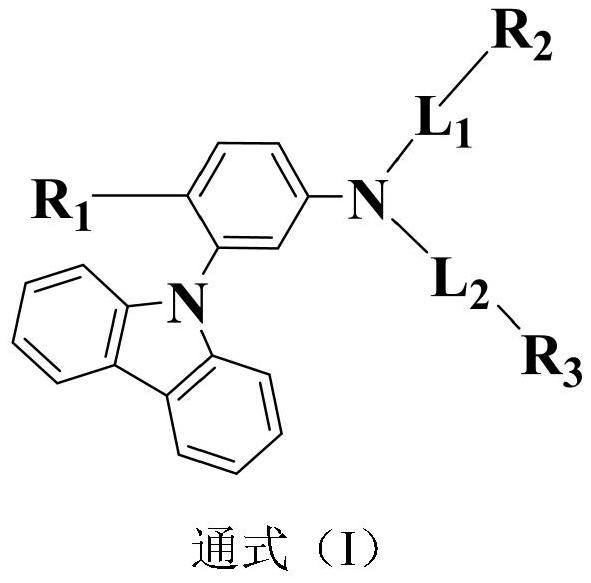
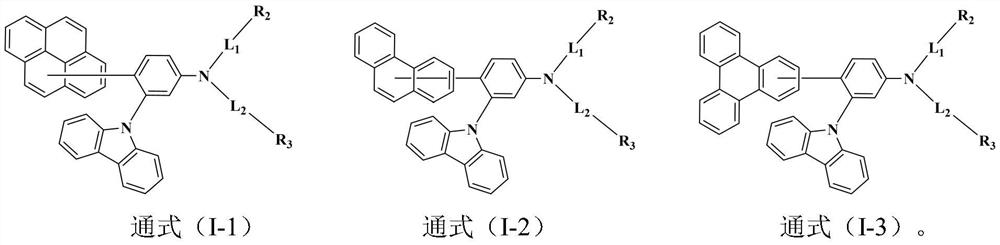
Triarylamine organic compound taking carbazole as core and application of triarylamine organic compound
A technology for organic compounds and triarylamines, applied in the field of triarylamine organic compounds, can solve problems such as differences, and achieve the effects of reducing the influence of deformation, improving the utilization rate of excitons, and improving the composite efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: the synthesis of intermediate B1:
[0045]
[0046] In a 250ml three-neck flask, under the protection of nitrogen, add 0.01mol raw material 1-1, 0.012mol raw material 2-1, 150ml toluene and stir to mix, then add 5×10 -5 molPd 2 (dba) 3 , 5×10 -5 mol P(t-Bu) 3 , 0.03mol sodium tert-butoxide, heated to 105°C, refluxed for 24 hours, sampled on a plate, and the reaction was complete; naturally cooled to room temperature, filtered, the filtrate was rotary evaporated to no fraction, passed through a neutral silica gel column, and the target product intermediate was obtained B1; HPLC purity 99.37%, yield 73.4%; Elemental analysis structure (molecular formula C 34 h 23 NO): Theoretical C, 88.48; H, 5.02; N, 3.03; Tested: C, 88.45; H, 5.01; N, 3.01. ESI-MS (m / z) (M+): The theoretical value is 461.18, and the measured value is 461.21.
[0047] The synthetic raw materials of intermediate B required in the embodiment are shown in Table 1:
[0048] Table 1 ...
Embodiment 2
[0052] Embodiment 2: the synthesis of intermediate C1:
[0053]
[0054] Under nitrogen atmosphere, add 0.01mol raw material 3-1, 0.025mol raw material 4-1, 0.03mol sodium tert-butoxide, 5×10 -5 mol Pd 2 (dba) 3 and 5×10 -5 mol of tri-tert-butylphosphine, then add 150ml of toluene to dissolve it, heat to 100°C, reflux for 24 hours, observe the reaction by TLC until the reaction is complete. Naturally cooled to room temperature, filtered, and the filtrate was rotary evaporated until there was no fraction. The resulting material was purified by silica gel column (petroleum ether as eluent) to obtain intermediate C-1. HPLC purity 98.18%, yield 82.6%; Elemental analysis structure (C 34 h 20 BrN) Theoretical: C, 78.17; H, 3.86; Br, 15.29; N, 2.68; Found: C, 78.17; H, 3.86; Br, 15.29; N, 2.68. ESI-MS (m / z) (M+): The molecular weight of the material is 521.08, and the measured molecular weight is 521.27.
[0055] The synthetic raw materials of intermediate C required in th...
Embodiment 3
[0058] Embodiment 3: the synthesis of compound 14:
[0059]
[0060] In a 250ml three-neck flask, under the protection of nitrogen, add 0.01mol intermediate B1, 0.012mol intermediate C1, 150ml toluene and stir to mix, then add 5×10 -5 molPd 2 (dba) 3 , 5×10 -5 mol P(t-Bu) 3 , 0.03mol sodium tert-butoxide, heated to 105°C, refluxed for 24 hours, sampled and plated, the reaction was complete; naturally cooled to room temperature, filtered, the filtrate was rotary evaporated to no fraction, passed through a neutral silica gel column, and the target product was obtained, HPLC The purity is 99.63%, and the yield is 79.4%. Elemental analysis structure (molecular formula C 68 h 42 N 2 O): Theoretical: C, 90.44; H, 4.69; N, 3.10; Tested C, 90.47; H, 4.65; N, 3.12. ESI-MS (m / z) (M+): Calculated 902.33, found 902.39. 1 H NMR (500MHz, Chloroform-d) δ8.22(dt,1H),8.17–8.10(m,2H),8.06–7.94(m,8H),7.87–7.76(m,3H),7.76(dt,1H ),7.69(d,1H),7.67–7.53(m,9H),7.53–7.37(m,7H),7.37–7.31(m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com