Clarifying process and quality control method of medicinal composition with function of repairing skin barrier
A quality control method, skin repair technology, applied in the direction of drug combination, medical preparations containing active ingredients, skin diseases, etc., to achieve the effect of repairing the skin barrier
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
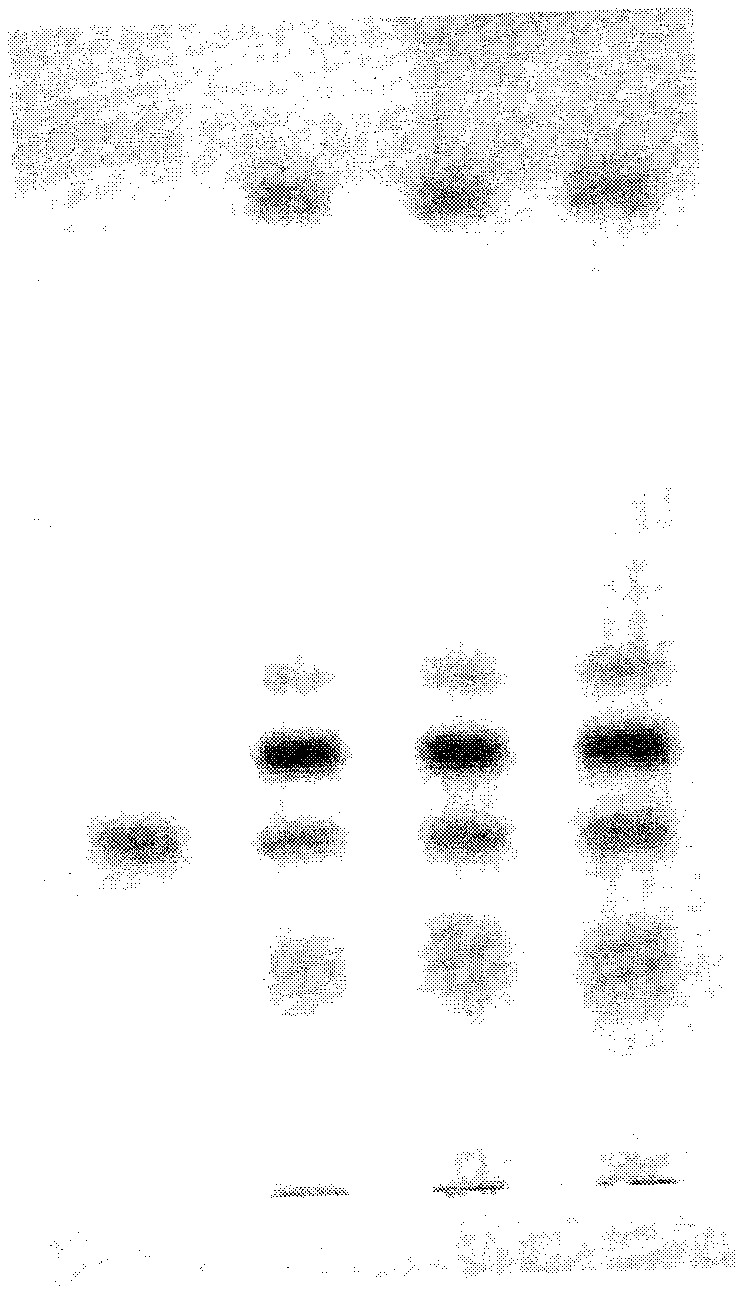
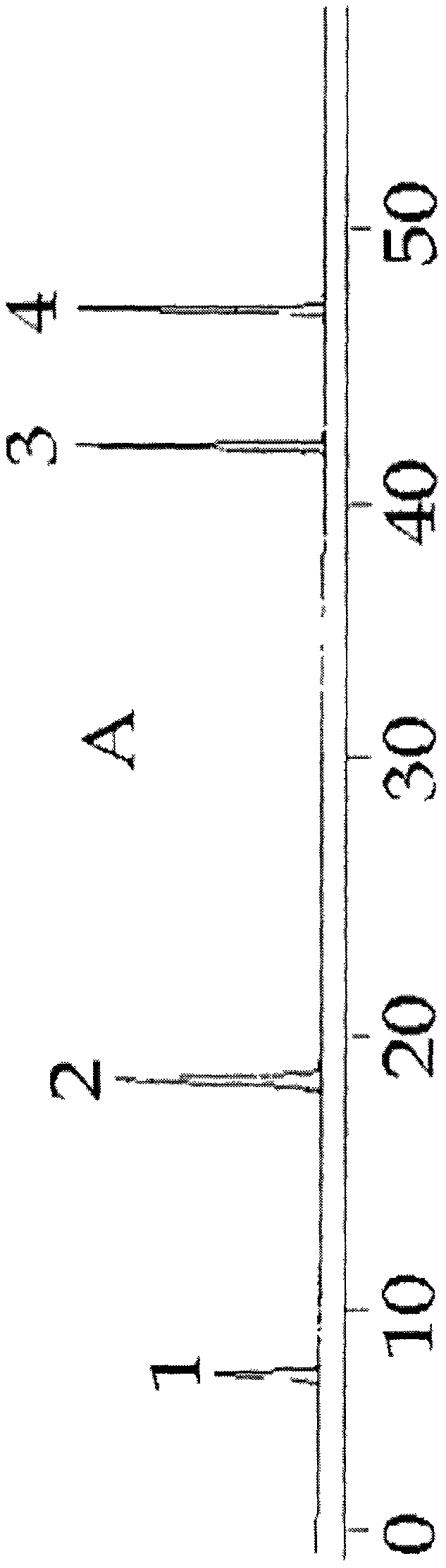
[0047] A clarification process and quality control method of a pharmaceutical composition with the effect of repairing the skin barrier of the present invention, the clarification process is carried out according to the following steps:
[0048] Clarification process and content of each component
[0049] (1) Preparation of clarifying agent: Type II ZTC1+1 natural clarifying agent consists of A and B components. Component A: Precisely measure 1.0g of component A, add a small amount of distilled water, stir to form a paste, then add distilled water to prepare 100mL of viscose, swell for 24 hours, and filter with double gauze. Component B: Precisely measure 1.0g of component B, add a small amount of 1% acetic acid solution by volume, stir to form a paste, then add 1% acetic acid solution to make 100mL viscose solution, swell for 24 hours, Filter with double gauze, that is, too.
[0050] (2) Adding order of clarifiers A and B: According to the instructions, ZTC natural clarifie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com