Preparation method of 1-(5-(2-fluorophenyl)-1-3-(3-methoxypropoxyl) benzene sulfonyl chloride)-1H-pyrrole-3-yl)-N-methyl amine
A technology of methoxypropoxy and methoxypropoxy, applied in 1‑(5‑(2‑fluorophenyl)‑1‑3‑(3‑methoxypropoxy)benzenesulfonyl chloride)‑ The preparation field of 1H-pyrrole-3-yl)-N-methylamine can solve the problems of slow onset of acid breakthrough at night, instability, influence of administration time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
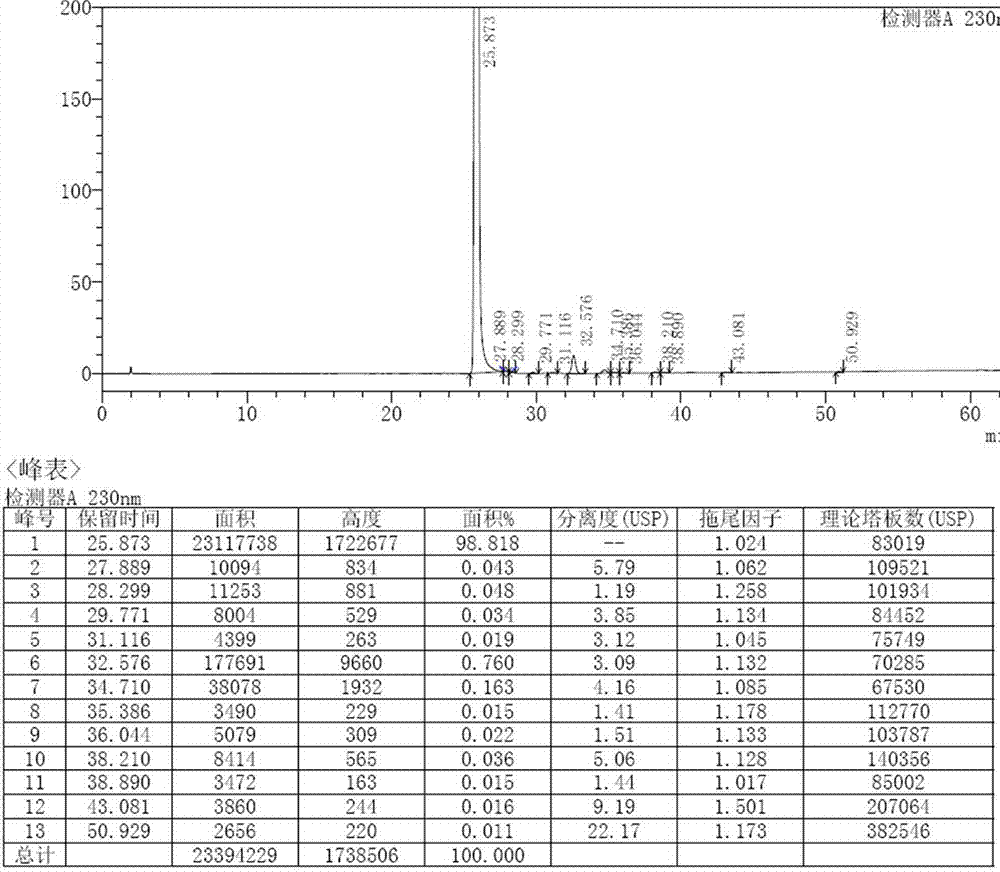
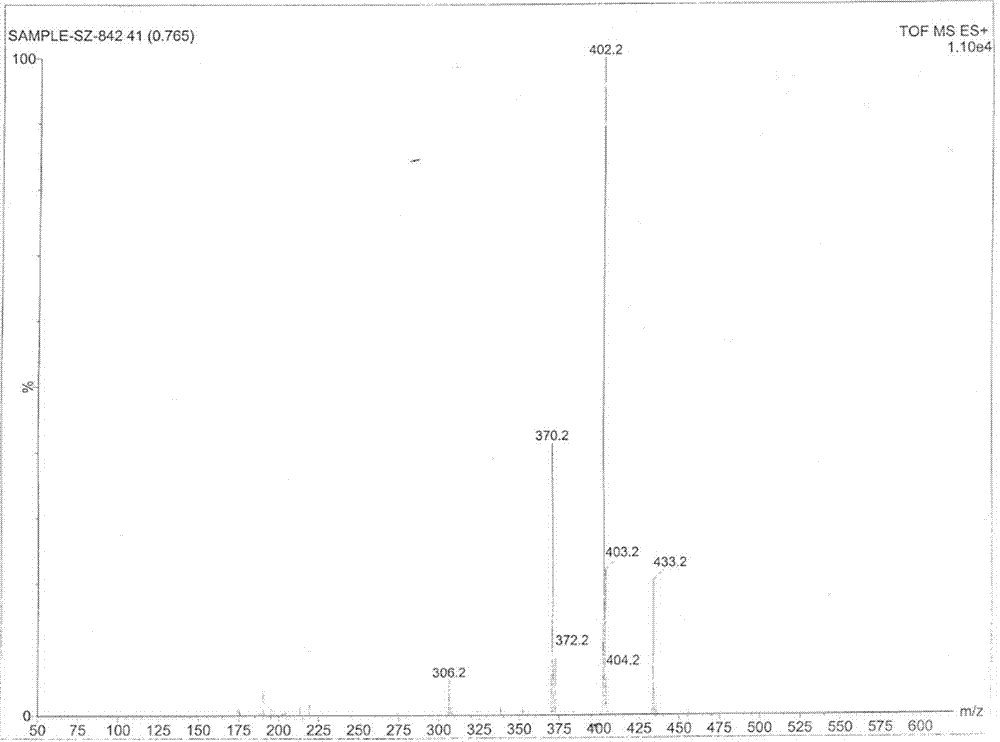
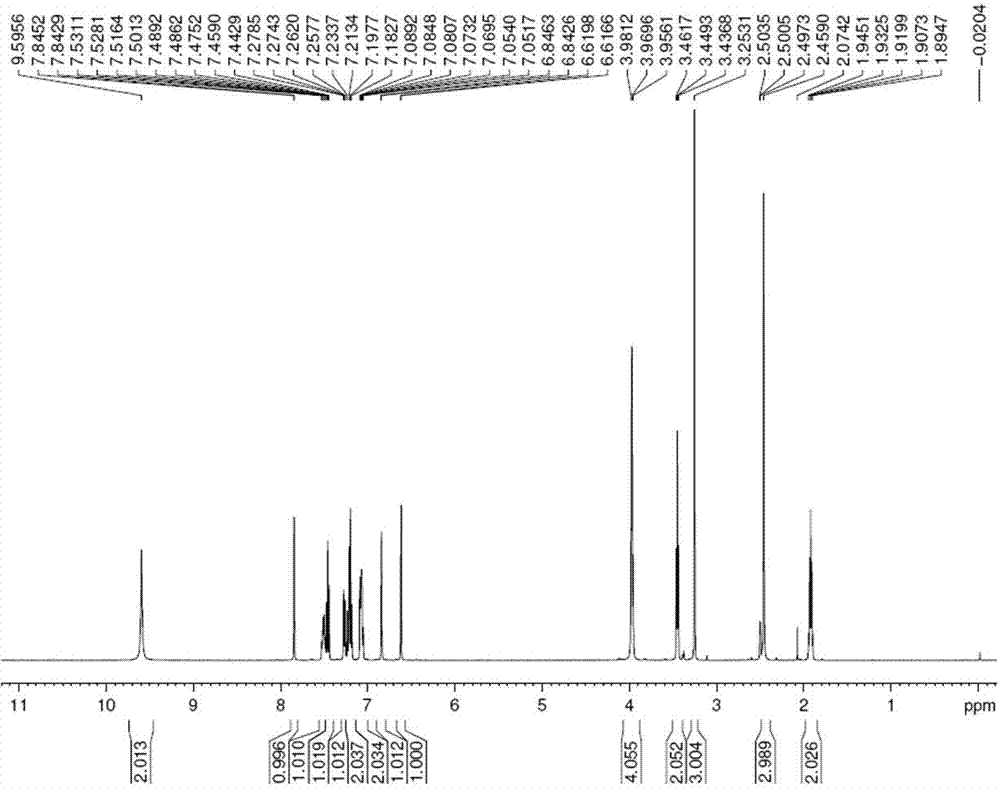
Image
Examples
Embodiment 1
[0020] Example 1: Preparation of 5-(2-fluorophenyl)-1-((3-(3-methoxypropoxy)benzenesulfonyl)-1H-pyrrole-3-carbaldehyde (compound 2)
[0021]
[0022] In a 10L reaction flask, add 60% NaH (300g, 7.62mol) to a solution of 1-1 (480g, 2.54mol) in THF (200ml), stir at -30°C for 25 min, add dropwise compound 1 (800g, 3.06 mol), stirred at -30°C for 10 min. The reaction solution was poured into 5L of ice water and extracted with ethyl acetate (2.5Lx3). The combined organic phase was dried, concentrated, added 1.5 ethanol and stirred for 1 h, suction filtered, and vacuum-dried to obtain a light yellow Solid 700g (66% yield).
Embodiment 2
[0023] Example 2: Preparation of 5-(2-fluorophenyl)-1-((3-(3-methoxypropoxy)benzenesulfonyl)-1H-pyrrole-3-carbaldehyde (compound 2)
[0024]
[0025] In a 2L reaction flask, dissolve compound 1-1 (95g, 0.5mol) in DMF (1000ml), add sodium tert-butoxide (96g, 1mol), stir for 25 min, and add compound 1 (158g, 0.6mol) dropwise , and stirred at 25°C for 1 hour. The reaction solution was cooled to room temperature, filtered with suction, concentrated to dryness under reduced pressure, and 80 g of a light yellow solid was obtained by column chromatography (yield 38.2%).
Embodiment 3
[0026] Example 3: Preparation of 5-(2-fluorophenyl)-1-((3-(3-methoxypropoxy)benzenesulfonyl)-1H-pyrrole-3-carbaldehyde (compound 2)
[0027]
[0028] In a 2L reaction flask, dissolve compound 1-1 (95g, 0.5mol) in acetonitrile (1000ml), add N, N-diisopropylethylamine (97g, 0.75mol), stir at room temperature for 20 min, drop Compound 1 (158g, 0.6mol) was stirred at room temperature for 1 hour. The reaction solution was cooled and then concentrated to dryness under reduced pressure. Column chromatography gave 68g of a light yellow solid (yield 32.4%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com