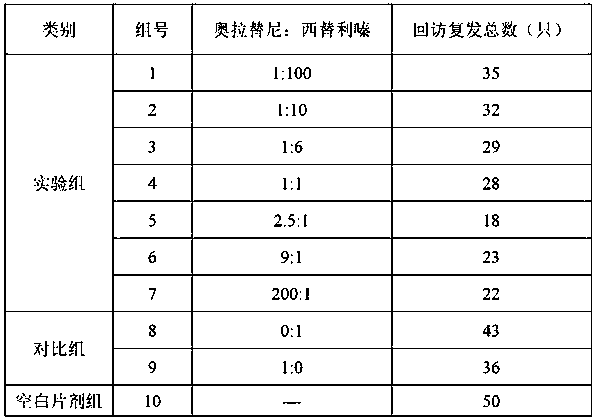
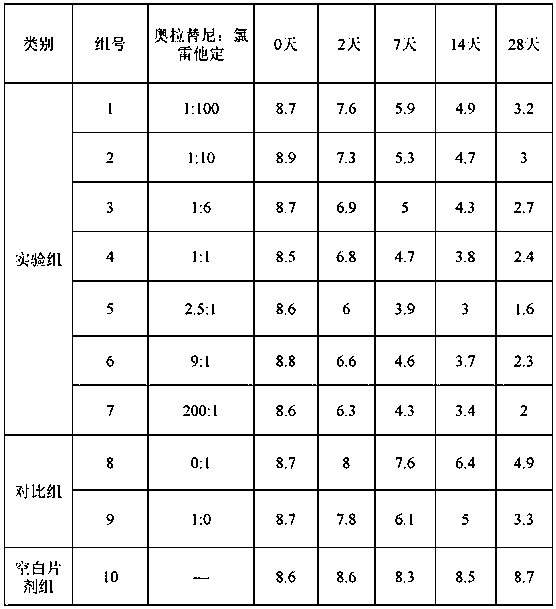
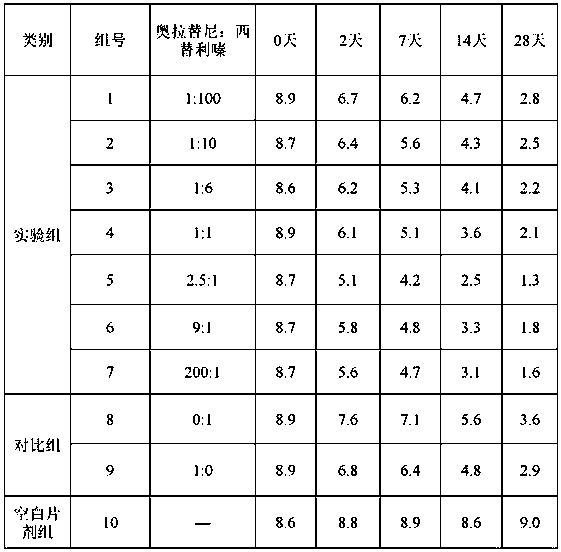
Compound olaparib composition preparation for pet allergic skin disease
A technology of oclatinib and its composition, which is applied in the field of veterinary medicine, can solve problems such as a narrow range of action, and achieve the effects of simple production process, convenient operation, flexible administration method and dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-10
[0040] The prescription (powder, 100 g) of the compound oclatinib composition for pet allergic skin diseases of the present invention is as follows.
[0041] Table 2. Example 1-10 formulations.
[0042]
[0043] The preparation method comprises the following steps: placing the powdered oclacitinib, anti-H1 receptor antagonist, beef flavor or fish meat flavor, and lactose in an oven, drying at 40°C for 2 hours, taking them out, and passing through a 120-mesh sieve respectively. Take an appropriate amount of lactose and grind it in a mortar to make the inner wall saturated, then pour it out, put oclacitinib and beef flavor (or fish meat flavor) in the mortar and grind them evenly, and gradually add anti-H1 receptors by equal volume mixing method Grind the antagonists fully, then gradually add the required lactose according to the method of equal volume incremental mixing, knead evenly, and finally divide it into single-dose powder by weight method, and pack it.
Embodiment 11-20
[0045] A single-dose prescription (tablet, 100g / 1000 tablets) of the compound oclatinib composition preparation for pet allergic skin diseases of the present invention is.
[0046] Table 3. Example 11-20 formulations.
[0047]
[0048] The preparation method includes the following steps: place the original drug powder oclatinib, anti-H1 receptor antagonist, filler, lubricant magnesium stearate and glidant micropowder silica gel in an oven for 2 h at 40°C, take them out, and separate Pass through a 120 mesh sieve. Take the prescribed amount of powder and mix thoroughly, and then directly press the powder into tablets.
Embodiment 21-30
[0050] The prescription (syrup, 100 mL) of a compound oclatinib composition preparation for pet allergic skin diseases of the present invention is as follows.
[0051] Table 4. Example 21-30 formulations.
[0052]
[0053] The preparation method includes the following steps: under the condition of normal temperature (25°C), the main drug oclatinib, the anti-H1 receptor antagonist, the flavoring agent beef flavor / fish flavor, and the preservative benzoic acid are dissolved in a certain amount of distilled water; Take the prescribed amount of sucrose, dissolve it in a certain amount of distilled water with stirring at room temperature, and filter until it is clean. Mix the two parts of the solution and stir evenly at room temperature.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com