Chimeric antigen receptor containing c3ar intracellular domain, lentiviral vector, expressing cell and drug
A chimeric antigen receptor, intracellular domain technology, applied in receptor/cell surface antigen/cell surface determinant, virus/phage, retroRNA virus, etc., can solve the unpredictable effect of chimeric antigen receptor How to improve the killing effect, improve the killing efficiency in vitro, and eliminate the effects of immunosuppression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Preparation of Plasmids Containing Anti-CD19 ScFv-4-1BB-CD3ζ-C3aR (CAR19C3aR)
[0032] The plasmid carrying the chimeric antigen receptor gene containing the C3aR intracellular domain of the present invention is prepared according to the following steps:
[0033] (1) Plasmid pUC57-CAR19 containing anti-CD19ScFv-4-1BB-CD3ζ(CAR19) was obtained by means of gene synthesis, molecular cloning and other means , namely CD19ScFv-4-1BB-CD3ζ.
[0034] (2) The obtained pUC57-CAR19 plasmid was digested by endonuclease Pmel and Sep1 to obtain the CAR19 gene, and then the CAR19 gene was connected to the lentiviral vector pWPXLd-GFP to construct pWPXLd-CAR19-GFP.
[0035] (3) The obtained pWPXLd-CAR19-GFP plasmid was digested with endonucleases NotI and SpeI to obtain the intracellular fragment 4-1BB-CD3ζ of the CAR19 gene.
[0036] (4) Using the cDNA of the tandem C3aR intracellular signaling domain of 4-1BB-CD3ζ as a template, four primers were constructed, and 4-1BB-CD3ζ...
Embodiment 2
[0039] Example 2 Lentiviral Packaging of CAR Plasmids
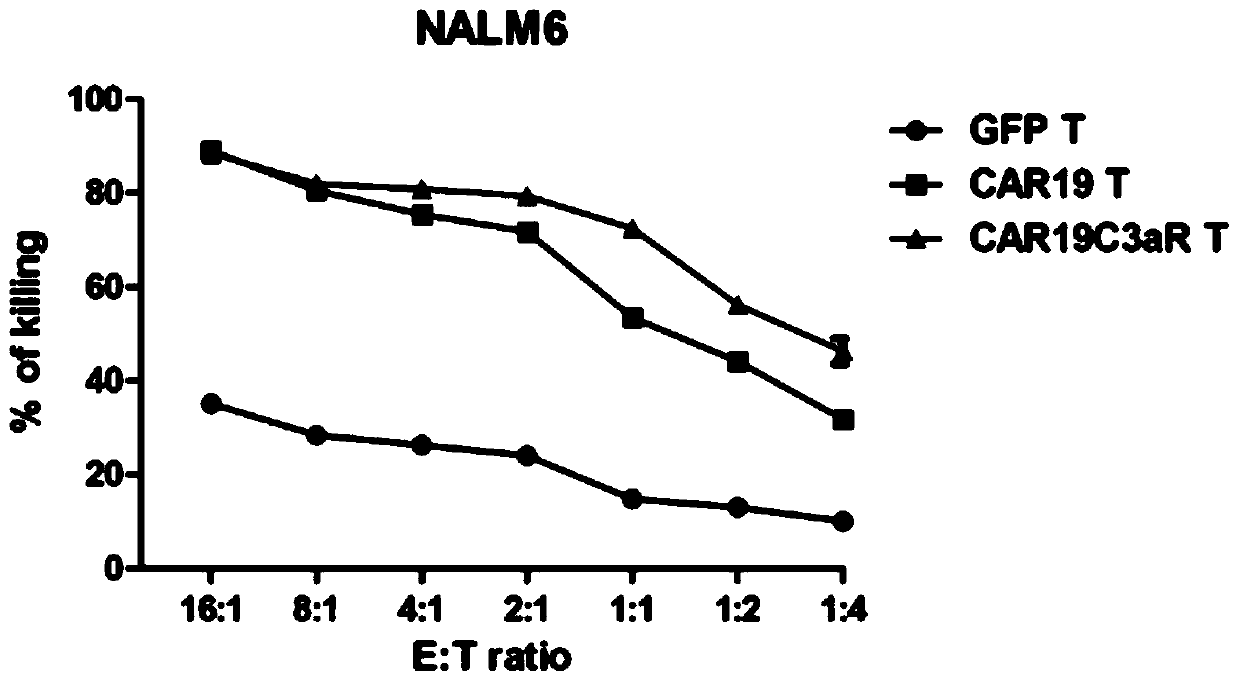
[0040] Three lentiviruses expressing GFP (blank control), CAR19-GFP (control) and CAR19C3aR-GFP were obtained by lentivirus packaging using the CAR plasmid of the present invention prepared in Example 1 and related control plasmids. In Examples 2 and 3, the CAR-containing plasmid is uniformly described as pWPXLd-CAR-GFP plasmid, and the CAR-overexpressing lentivirus is uniformly described as CAR lentivirus.
[0041] Specific steps are as follows:
[0042] (1) Cultivate 293T cells in a 10cm petri dish, the medium is: DMEM high glucose medium + 10% FBS (fetal bovine serum) + 1% double antibody (100 × penicillin-streptomycin mixed solution);
[0043] (2) When the density of 293T cells in the 150mm culture dish reaches 80-90%, replace the medium: DMEM high glucose medium + 1% FBS + 1% double antibody;
[0044] (3) After replacing the medium and culturing for 2-6 hours, the two plasmids pWPXLd-CAR-GFP (that is, containing CA...
Embodiment 3
[0052] Example 3 Infection of human T cells using packaged CAR virus
[0053] (1) Separation and purification of T cells: Mononuclear cells in blood were separated by Ficoll density gradient method, and red blood cells were removed by lysing with red blood cell lysate, and then T cells were sorted by MACS Pan-T magnetic beads;
[0054] (2) Dilute the sorted T cells with medium (AIM-V medium + 5% FBS + penicillin 100U / ml + streptomycin 0.1 mg / ml) to a cell concentration of 2.5×10 6 pcs / ml for use;
[0055] (3) T cells are stimulated by magnetic beads coated with CD2, CD3 and CD28 antibodies (product source: Miltenyi, Germany), that is, the coated magnetic beads and T cells are mixed in a ratio of 1:2, and the final density of T cells should be 5 ×10 6 pcs / ml / cm2. After mixing, the cells were placed in a 37°C, 5% CO2 incubator for stimulation for 48 hours.
[0056] (4) Lentivirus-transfected T cells: remove the magnetic beads in the activated T cell-magnetic bead mixture by ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com