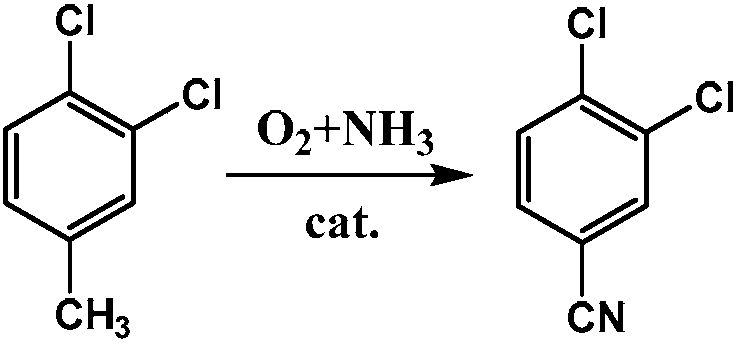
Synthesis method of 3,4-dichlorobenzonitrile
The technology of a dichlorobenzonitrile and a synthesis method, which is applied in the preparation of pharmaceutical intermediates and the field of pesticides, can solve the problems of high price, rare starting materials, large supply of raw materials, etc., and achieves low cost of raw materials, mild and controllable reaction conditions, and low reaction conditions. short step effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Bromination
[0034] Mix 26.2g of 1,2-dichlorobenzene with 2.75g of iron powder, heat to 45°C, slowly add 26g of bromine dropwise at a rate of 0.2mL / min, keep the reaction for 2 hours after the dropwise addition, and add sodium sulfite solution after the reaction is completed Wash with dichloromethane, get 41.6g of crude product after precipitation, containing 5.5% of 1,2-dichlorobenzene in the crude product, 2.1% of isomer 2,3-dichlorobromobenzene, high boiling (mainly dibromo or Polybrominated products, the same below) 0.6%, rectification, collect -0.09 ~ -0.1MPa 100 ~ 110 ℃ fraction recovery, collect -0.09 ~ -0.1MPa 160 ~ 165 ℃ fraction, that is, 3,4- 33.2 g of refined dichlorobromobenzene, the purity of 3,4-dichlorobromobenzene is 98.5%, and the molar yield is 90.3%.
[0035] (2) Cyanide
[0036] Mix and heat 5.4g cuprous cyanide, 0.1g L-proline and 25g DMF to 128°C, add dropwise 3,4-dichlorobromobenzene diluent (11.3g 3,4-dichlorobromobenzene and 10g DMF Mix...
Embodiment 2
[0038] (1) Bromination
[0039] Mix 26.2g of 1,2-dichlorobenzene with 2.75g of iron powder, heat to 75°C, slowly add 26g of bromine dropwise at a rate of 0.25mL / min, keep the reaction for 2 hours after the dropwise addition, and add sodium sulfite solution after the reaction is completed Extract and wash with dichloromethane, get 41.3g of crude product after desolvation, the crude product contains 4.8% of 1,2-dichlorobenzene, 2.0% of isomer 2,3-dichlorobromobenzene, high boiling 5.2%, rectification, collection -0.09~-0.1MPa 100~110℃ distillate recovery and apply mechanically, collect -0.09~-0.1MPa 160~165℃ distillate to obtain 31.5g of 3,4-dichlorobromobenzene refined product, 3,4-dichlorobromobenzene The purity of benzene is 98.3%, and the molar yield is 85.7%.
[0040] (2) Cyanide
[0041] Mix and heat 5.4g cuprous cyanide, 0.1g L-proline and 25g DMF to 150°C, add dropwise 3,4-dichlorobromobenzene diluent (11.3g 3,4-dichlorobromobenzene and 10g DMF Mixed solution of the m...
Embodiment 3
[0043] (1) Bromination
[0044] Mix 26.2g of o-dichlorobenzene and 0.59g of ferric chloride, heat to 45°C, slowly add 26g of bromine dropwise at a rate of 0.3mL / min, keep the reaction for 2 hours after the dropwise addition, after the reaction, add sodium sulfite solution and Dichloromethane was extracted and washed, and 41.1g of crude product was obtained after precipitation. The crude product contained 5.0% of 1,2-dichlorobenzene, 1.0% of isomer 2,3-dichlorobromobenzene, and 2.6% of high boiling point. It was rectified and collected- 0.09~-0.1MPa 100~110℃ distillate recovery, collect -0.09~-0.1MPa 160~165℃ distillate to obtain 33.1g of 3,4-dichlorobromobenzene refined product, 3,4-dichlorobromobenzene The purity is 98.5%, and the molar yield is 90.0%.
[0045] (2) Cyanide
[0046] Mix and heat 5.4g cuprous cyanide, 0.1g 1,10-phenanthroline hydrate and 25g DMF to 128°C, add dropwise 3,4-dichlorobromobenzene diluent (11.3g 3,4-dichlorobromobenzene mixed solution with 10g DM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com