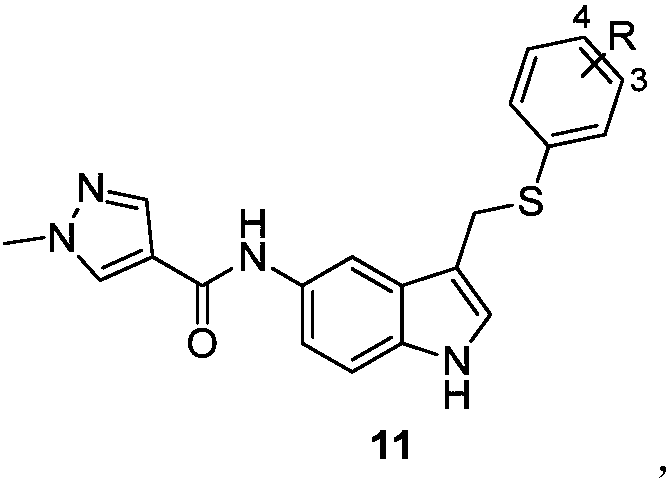
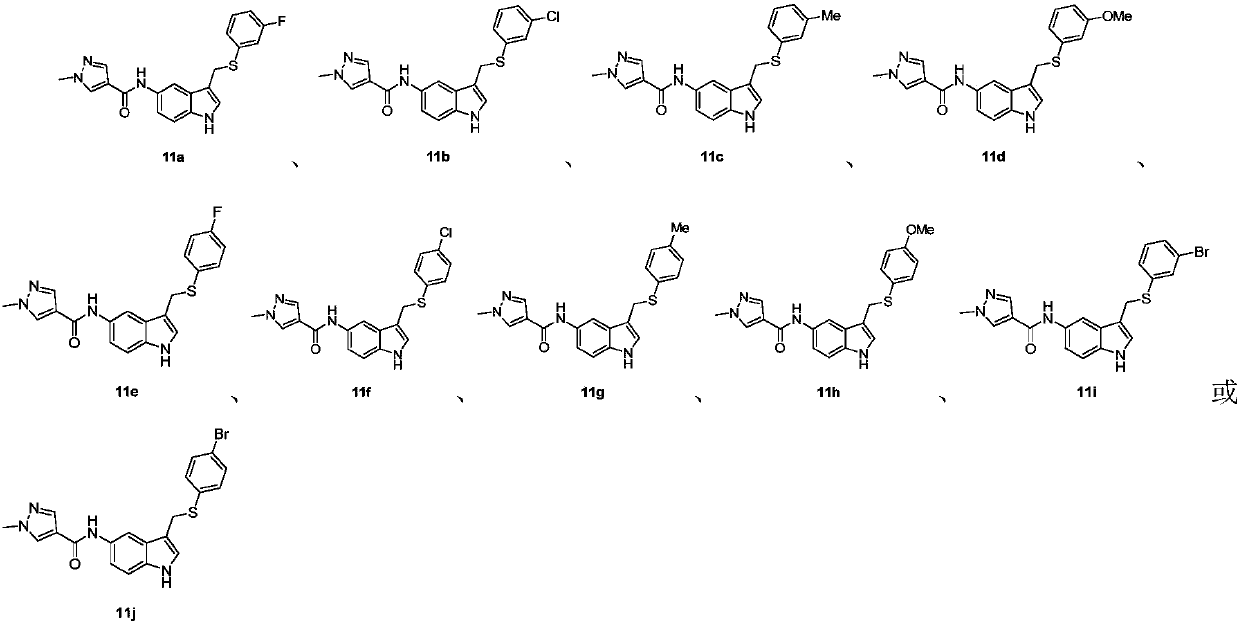
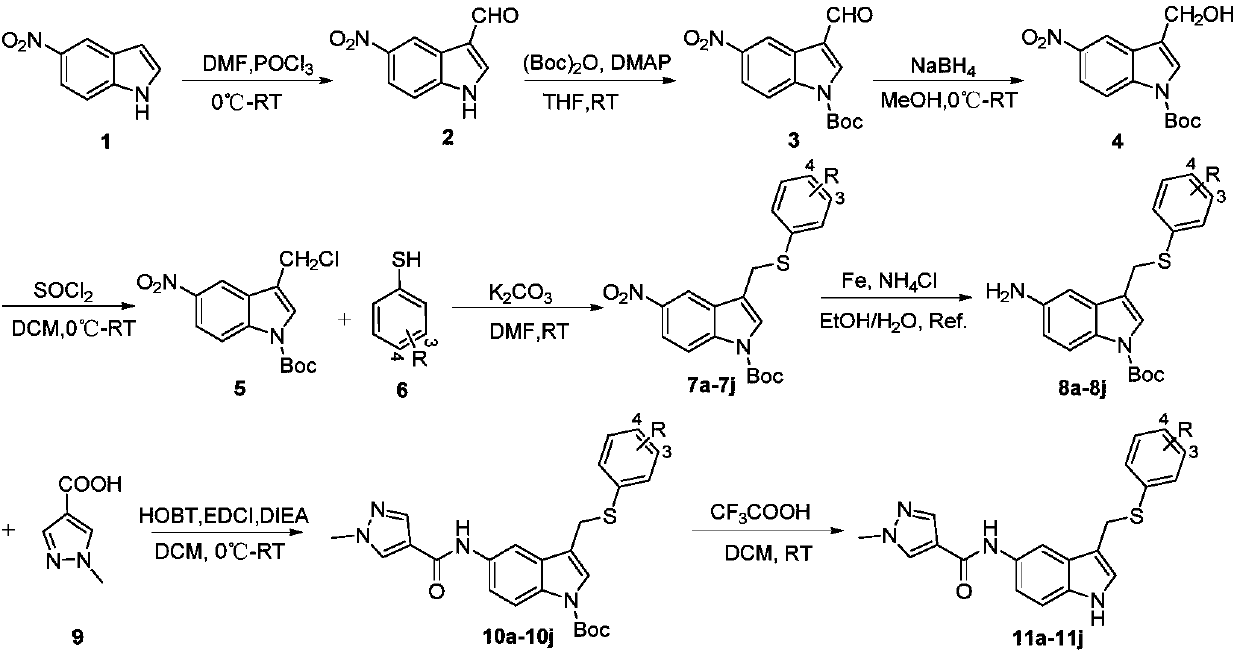
Novel 3,5-di-substituted 1H-indole derivative and synthesis and application thereof
A metabolite and extraction technology, applied in drug combinations, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problems of poor patient tolerance, high toxicity, and large individual differences in targeted drugs, and achieve good inhibition. Active, non-toxic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The synthesis of embodiment 1 target compound
[0048] 1. Synthesis of Intermediate 2
[0049]
[0050] At 0°C, POCl 3 (9.2ml, 98.5mmol) was added dropwise to DMF (9.5ml, 123.1mmol), and after stirring for 0.5h, 5-nitro-1H-indole (compound 1, 2.0g, 12.3mmol) was added to the reaction solution, Then react at room temperature for 1.5h. Then the reaction solution was poured into a large amount of ice water and adjusted to pH 7 with 6N NaOH, then extracted with ethyl acetate (×3), and concentrated to obtain 2.34 g of gray solid with a yield of 98% and a purity of 95.2%. LCMS m / z:191.0[M+H] + .
[0051] 2. Synthesis of Intermediate 3
[0052]
[0053] Compound 2 (2.34g, 12.3mmol), di-tert-butyl dicarbonate (2.95g, 13.5mmol), and 4-dimethylaminopyridine (75.8mg, 0.62mmol) were added to 50ml of tetrahydrofuran, and reacted at room temperature After 3 hours, it was concentrated and recrystallized from ethyl acetate to obtain 3.3 g of a light yellow solid with a yiel...
experiment example 1
[0165] 1. Experimental materials
[0166] DMEM: DMEM medium
[0167] 10% FBS: Fetal Bovine Serum
[0168] DMSO: dimethyl sulfoxide
[0169] Experimental tumor cell lines: pancreatic cancer BxPC-3 cell line, normal human liver cells HL-7702
[0170] 2. Cell preparation and processing
[0171] First, the cell lines were inoculated into 96-well plates containing DMEM+10% FBS medium, at 37°C, 5% CO 2 conditions overnight. On the next day, add the compound to be tested at gradient concentrations diluted in the culture medium and a blank control for 72 hours, then add 20 μl of MTT reagent at a concentration of 5 mg / mL to each well, and continue to incubate for 2-4 hours. After the formation of formazan, the supernatant was sucked off, and then 150 μL of DMSO was added to each well, shaken to fully dissolve the formed formazan, and the absorbance value (OD570) at a wavelength of 570 nm was measured with a microplate reader.
[0172] Finally, according to the inhibition rate=(bl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com