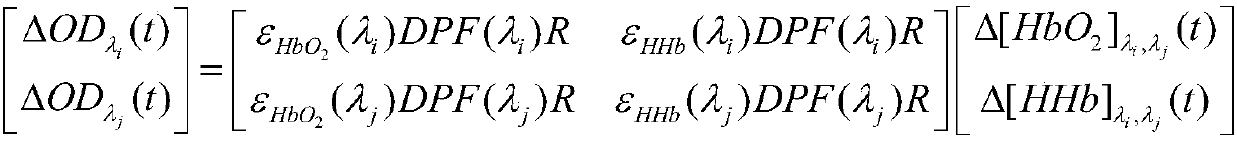Near-infrared brain function signal processing method based on differential pathlength factor estimation
A differential path and signal processing technology, applied in medical science, telemetry patient monitoring, sensors, etc., can solve the problems of measurement error interference, near-infrared brain function activity response signal measurement and extraction accuracy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
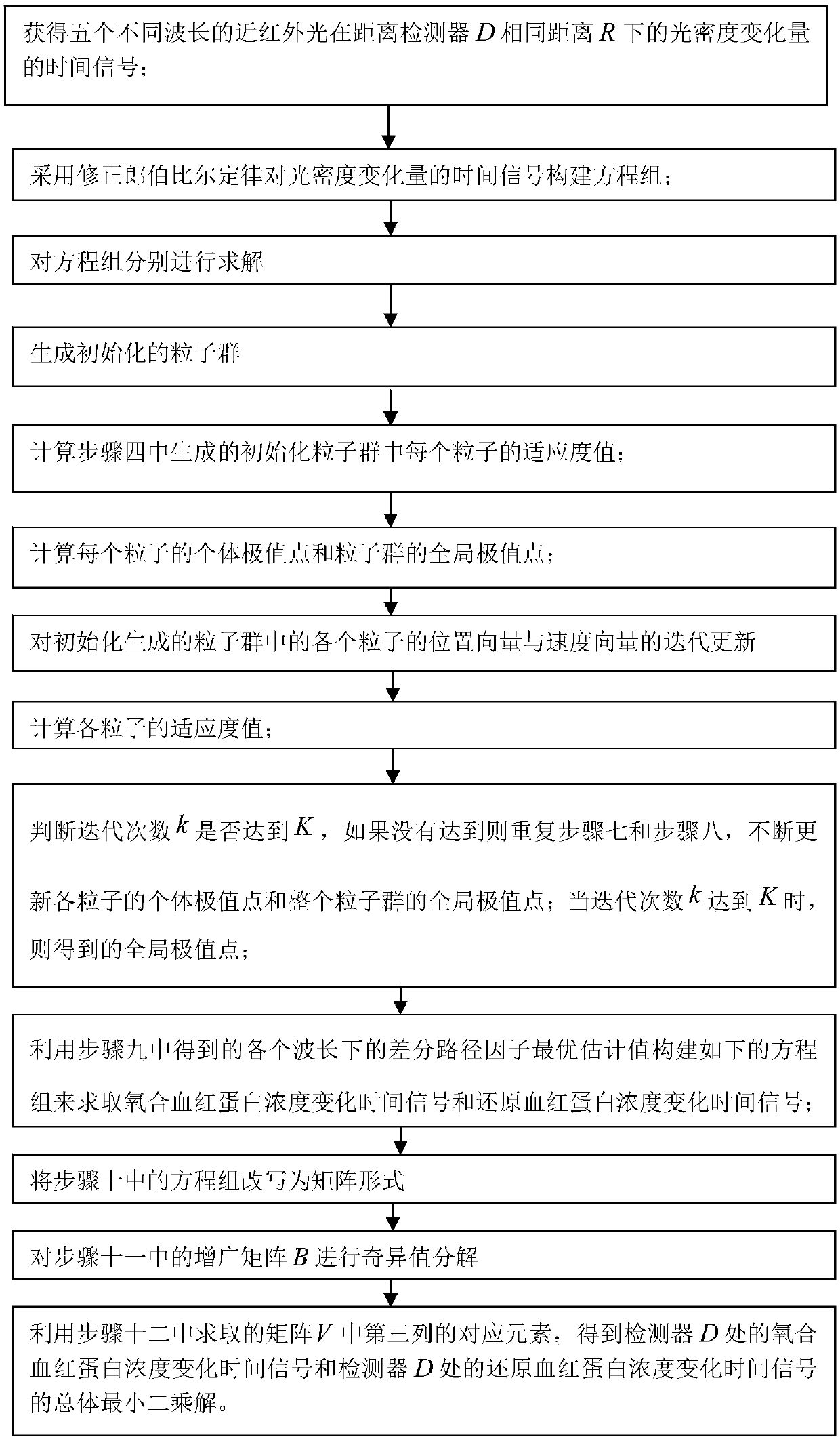
Method used
Image
Examples
specific Embodiment approach 1
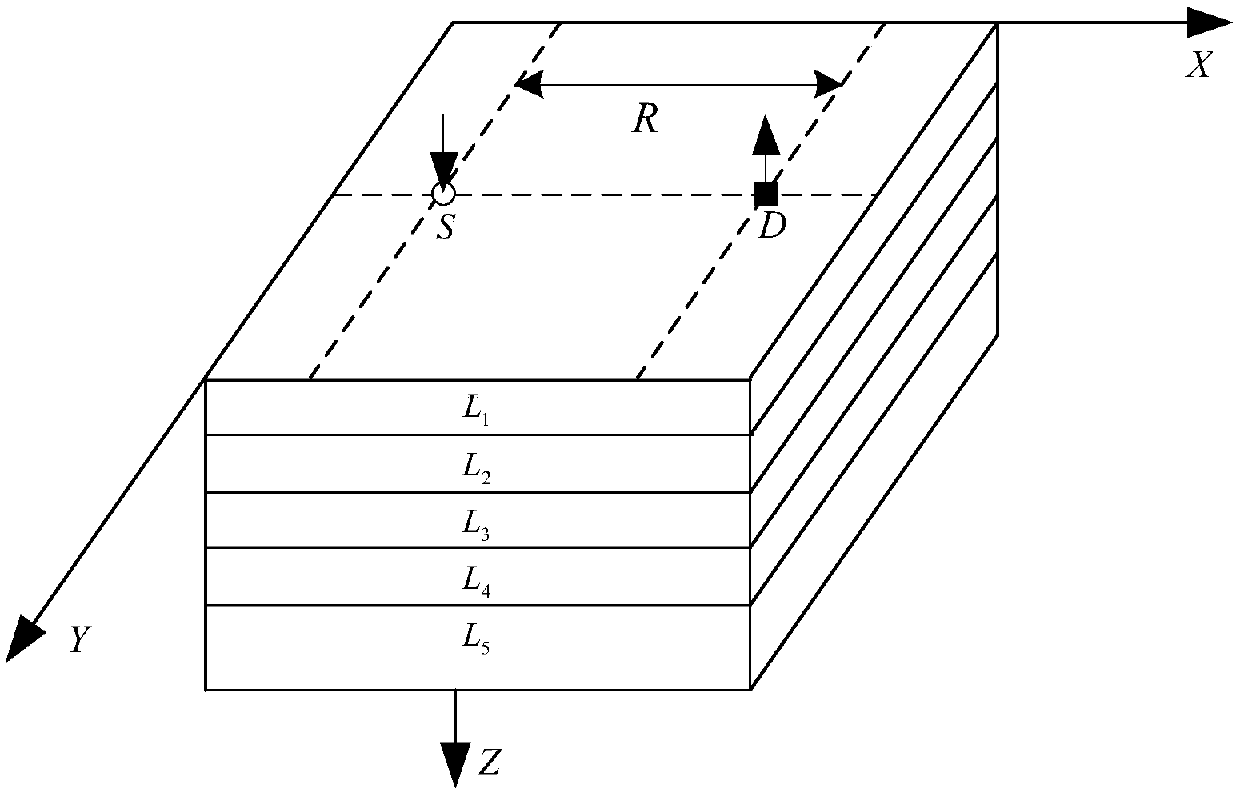
[0068] Specific implementation mode one: combine figure 1 , figure 2 Describe this embodiment, the method for processing near-infrared brain function signals based on differential path factor estimation in this embodiment is specifically implemented according to the following steps:
[0069] Step 1: Place a near-infrared probe composed of a five-wavelength light source S and a detector D on the surface of the brain tissue to be tested. The linear distance between the five-wavelength light source S and the detector D is R, and the five-wavelength light source S emits The wavelength of near-infrared light is λ 1 , lambda 2 , lambda 3 , lambda 4 and lambda 5 , the detector D is used to obtain the diffuse reflection light intensity in the quiet state of the brain and the diffuse reflection light intensity in the brain-evoked excitation state, so as to obtain the optical density of five different wavelengths of near-infrared light at the same distance R from the detector D T...
specific Embodiment approach 2
[0125] Specific embodiment 2: The difference between this embodiment and specific embodiment 1 is that in step 10, the optimal estimated value of the differential path factor at each wavelength obtained in step 9 is used to construct the following equations to obtain oxyhemoglobin concentration change time signal and reduced hemoglobin concentration change time signal;
[0126] The specific equations can be expressed as follows:
[0127]
[0128] Among them, ε HHb (λ 1 ) is the wavelength λ of the near-infrared light emitted by the light source S 1 The extinction coefficient of reduced hemoglobin; ε HHb (λ 2 ) is the wavelength λ of the near-infrared light emitted by the light source S 2 The extinction coefficient of reduced hemoglobin; ε HHb (λ 3 ) is the wavelength λ of the near-infrared light emitted by the light source S 3 The extinction coefficient of reduced hemoglobin; ε HHb (λ 4 ) is the wavelength λ of the near-infrared light emitted by the light source S...
specific Embodiment approach 3
[0130] Specific embodiment three: the difference between this embodiment and one of the specific embodiments one to two is that in the step 13, the corresponding element in the third column in the matrix V obtained in the step 12 is used to obtain the detector D. The overall least squares solution of the oxygenated hemoglobin concentration change time signal and the reduced hemoglobin concentration change time signal at the detector D; respectively expressed as:
[0131]
[0132]
[0133] Among them, Δ[HbO 2 ] TLS (t) is the overall least squares solution of the oxygenated hemoglobin concentration change time signal at the detector D; Δ[HHb] TLS (t) is the overall least squares solution of the reduced hemoglobin concentration change time signal at the detector D.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com