Ethylenediamine cationic albumin antitumor nanoparticle as well as preparation method and application thereof
A cationization and ethylenediamine cationization technology, which is applied in the field of medicine, can solve the problems of low encapsulation efficiency and low drug loading rate, and achieve the effects of improving solubility, high safety, and good tumor targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
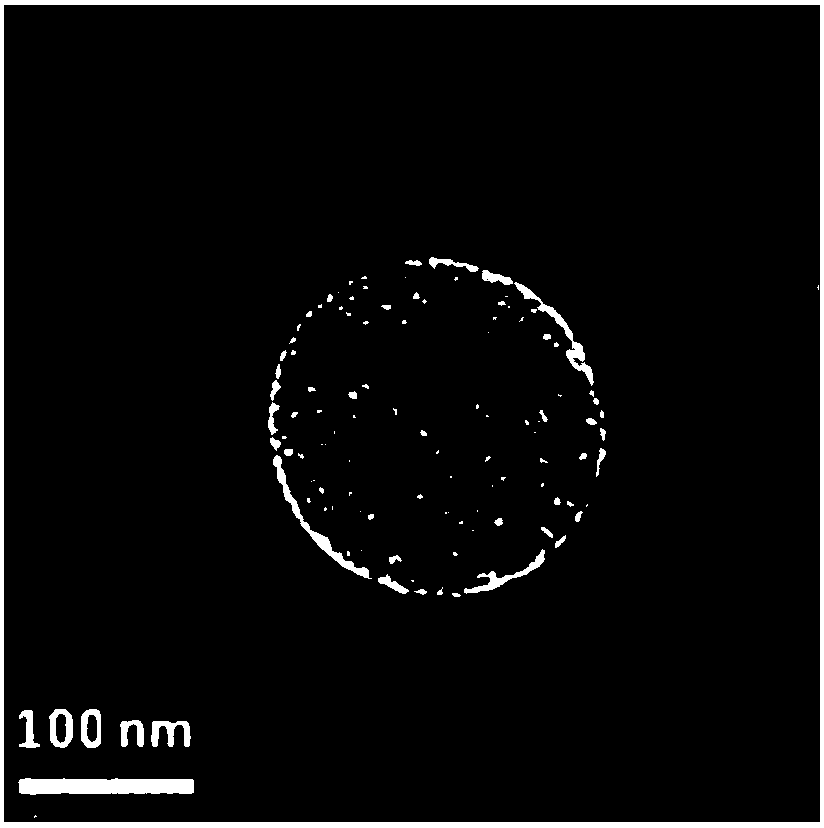
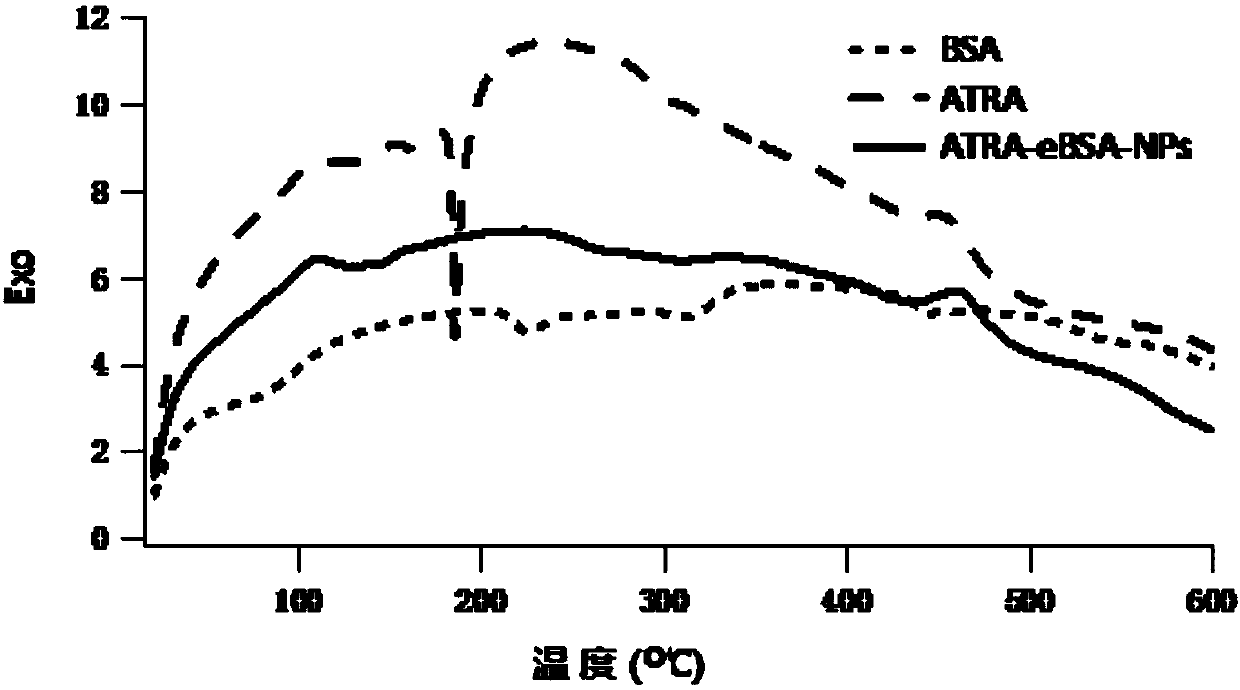
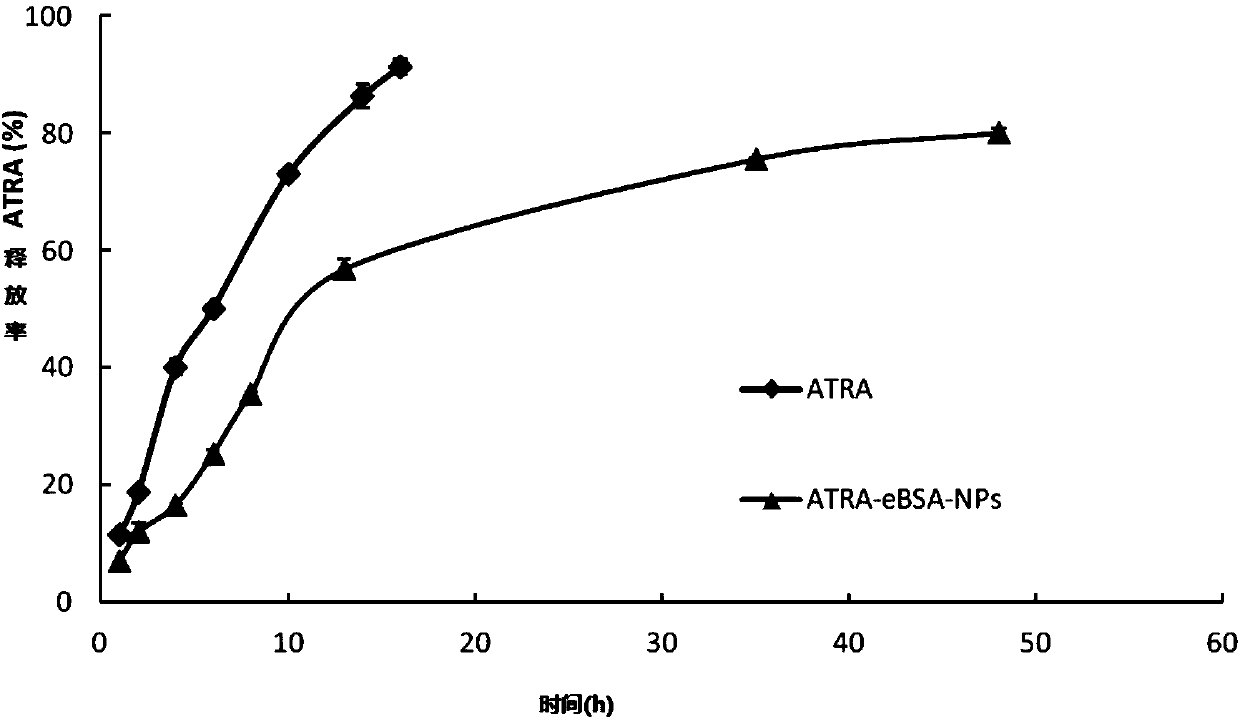
[0046] Preparation of ATRA-eBSA-NPs: Weigh 4.5mg of ATRA into a 1.5ml centrifuge tube, add 0.9ml of absolute ethanol, fully dissolve to obtain the drug-containing organic phase (5mg / ml); weigh 50mg of eBSA and purify it with 10ml Dissolved in water and placed in a glass vial as the water phase; inject the organic phase into the water phase, and immediately use a cell sonicator to sonicate in an ice bath, with an ultrasonic power of 50W, supersonic for 5s and stop for 5s, and sonicate for 20min Then the solution in the vial is transferred to a high-pressure homogenizer, and the high-pressure homogenization is performed under 18000psi pressure for 15 cycles; then the solution in the vial is transferred to a 100ml round-bottomed flask, and the organic solution is removed by rotary evaporation in a 35°C water bath, protected from light, and decompressed. solvent to obtain ATRA-eBSA-NPs solution. The appearance of the solution is light yellow, transparent and opalescent, and there ...
Embodiment 2
[0049] Preparation of ATRA-eBSA-NPs: Weigh 10mg of ATRA into a 5ml centrifuge tube, add 2ml of absolute ethanol, fully dissolve to obtain a drug-containing organic phase (5mg / ml); weigh 100mg of eBSA, dissolve it in 10ml of purified water and place Put it in a glass vial as the water phase; inject the organic phase into the water phase, and immediately use a cell sonicator to sonicate it in an ice bath with an ultrasonic power of 50W, supersonicate for 5s and stop for 5s, and sonicate for 20min in total; then Transfer the solution in the vial to a high-pressure homogenizer, and homogenize under high pressure for 15 cycles at a pressure of 18,000 psi; then transfer the solution in the vial to a 100ml round-bottomed flask, and remove the organic solvent by rotary evaporation in a 35°C water bath, protected from light, under reduced pressure, that is ATRA-eBSA-NPs solution was obtained. The appearance of the solution is light yellow, transparent and opalescent, and there is Tynda...
Embodiment 3
[0052] Preparation of ATRA-eBSA-NPs: Weigh 50mg of ATRA into a 10ml centrifuge tube, add 10ml of absolute ethanol, fully dissolve to obtain a drug-containing organic phase (5mg / ml); weigh 500mg of eBSA and dissolve it in 50ml of purified water Put it in a glass vial as the water phase; inject the organic phase into the water phase, and immediately use a cell sonicator to sonicate it in an ice bath with an ultrasonic power of 50W, supersonicate for 5s and stop for 5s, and sonicate for 20min in total; then Transfer the solution in the vial to a high-pressure homogenizer, and homogenize under high pressure for 15 cycles at a pressure of 18,000 psi; then transfer the solution in the vial to a 100ml round-bottomed flask, and remove the organic solvent by rotary evaporation in a 35°C water bath, protected from light, under reduced pressure, that is ATRA-eBSA-NPs solution was obtained. The appearance of the solution is light yellow, transparent and opalescent, and there is Tyndall ph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com