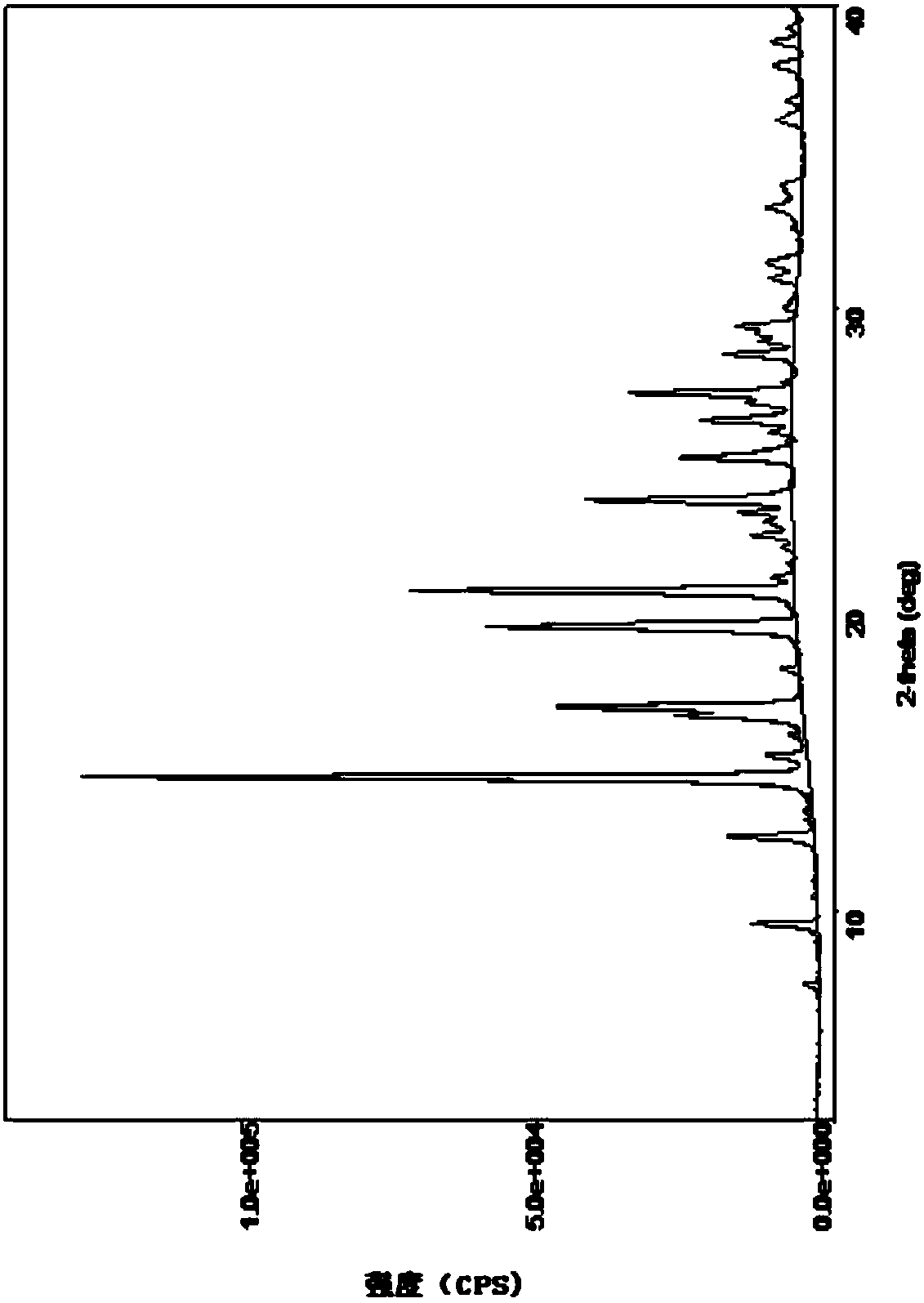
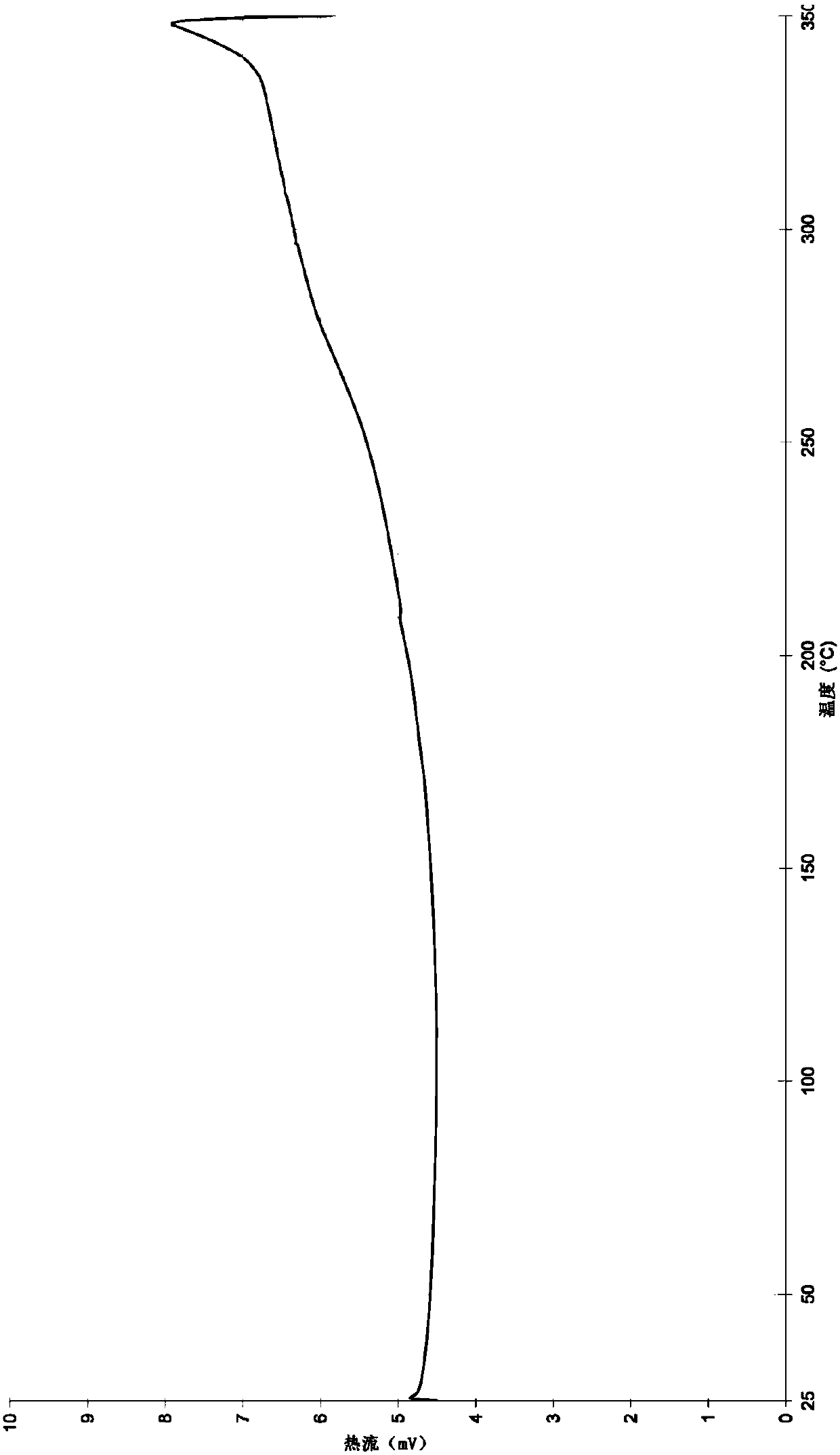
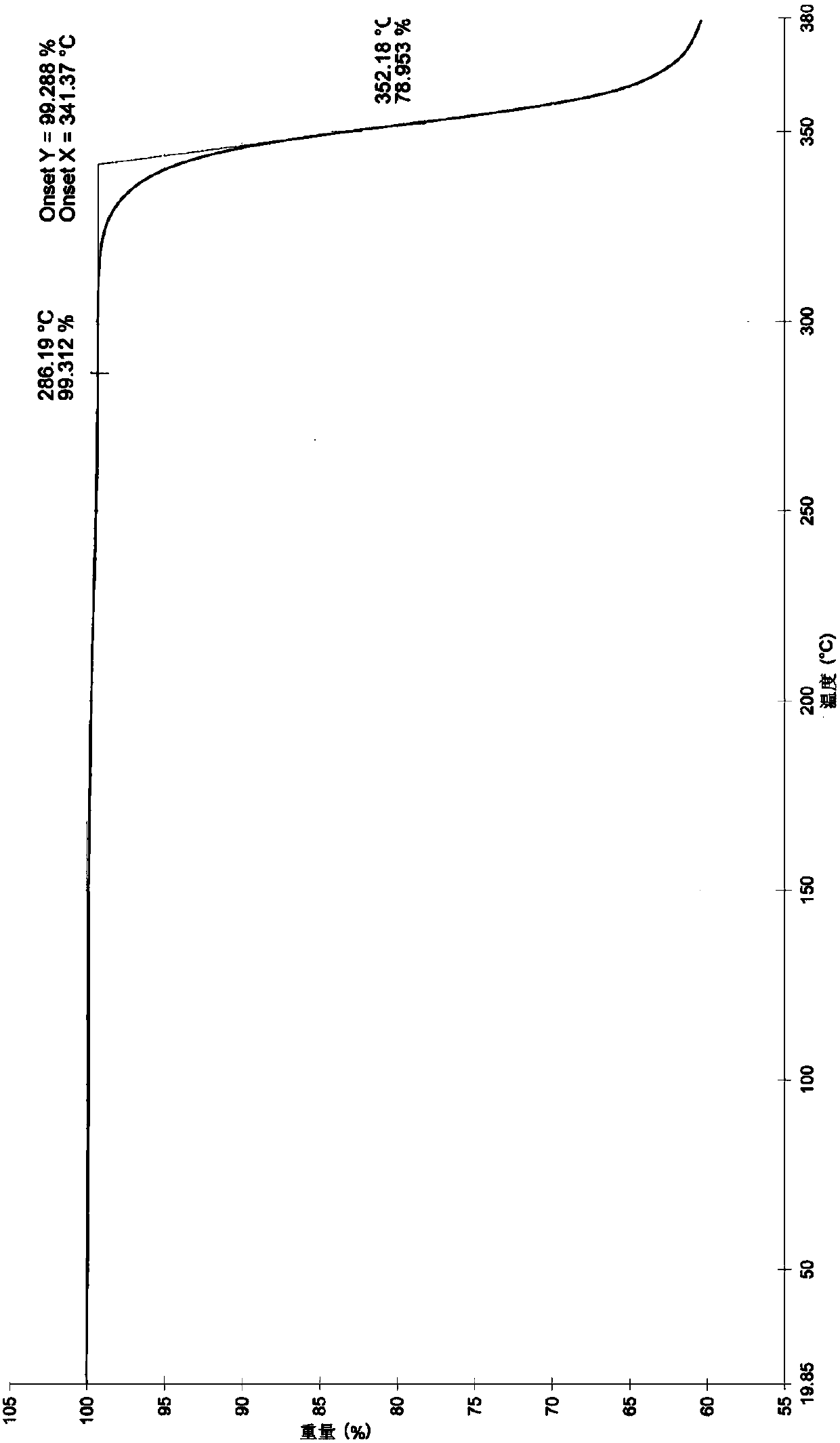
Crystal form of 3-(4-(1,1,1,3,3,3-hexafluoro-tert-butyl ester)benzyl)-2-keto-2H-benzopyran-4-sodium alkoxide, and preparation thereof
A technology of benzopyran and crystal form, which is applied to medical preparations containing active ingredients, blood diseases, extracellular fluid diseases, etc. Medicinal requirements and other issues, to achieve the effect of low cost, simple and easy-to-obtain solvent, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: 3-(4-(1,1,1,3,3,3-hexafluorotert-butylcarboethoxy)benzyl)-2-keto-2H-benzopyran-4-ol sodium crystal Form A preparation method
[0037]2g of 4-hydroxy-2-keto-3-(4-(1,1,1,3,3,3-hexafluoro-tert-butylcarboxylate) benzyl)-2H-benzopyran (4.3mmol) Dissolve 0.46g of sodium tert-butoxide (4.7mmol) in 30ml of acetone, react at -30 to 30°C for 3h, filter, evaporate the filtrate to dryness to obtain a pale yellow solid, dissolve it in 6ml of ethyl acetate, raise the temperature to 40°C, and add 40ml of Isooctane was crystallized to obtain crystal form A, and the sodium content was 95%-110% as detected by atomic method.
Embodiment 2
[0038] Example 2: 3-(4-(1,1,1,3,3,3-hexafluorotert-butylcarboethoxy)benzyl)-2-keto-2H-benzopyran-4-ol sodium crystal Form A preparation method
[0039] 3g of 4-hydroxy-2-keto-3-(4-(1,1,1,3,3,3-hexafluoro-tert-butylcarboxylate) benzyl)-2H-benzopyran (6.5mmol) Dissolve 0.31g of sodium hydroxide (7.8mmol) in 30ml of tetrahydrofuran, react at -30 to 30°C for 3 hours, filter, and evaporate the filtrate to dryness to obtain a light yellow solid, which is dissolved in 30ml of ethyl acetate, and crystallized by dropping 50ml of isooctane. The temperature was raised to 70° C. to obtain the crystal form A, and the sodium content was 95% to 110% as detected by the atomic method.
Embodiment 3
[0040] Example 3: 3-(4-(1,1,1,3,3,3-hexafluorotert-butylcarboethoxy)benzyl)-2-keto-2H-benzopyran-4-ol sodium crystal Form A preparation method
[0041] 5g of 4-hydroxy-2-keto-3-(4-(1,1,1,3,3,3-hexafluoro-tert-butylcarboxylate) benzyl)-2H-benzopyran (6.5mmol) Dissolve 0.48g of sodium hydroxide (7.8mmol) in 30ml of ethyl acetate, react at -30 to 30°C for 3h, filter, evaporate the filtrate to dryness to obtain a pale yellow solid, dissolve it in 30ml of methyl isobutyl ketone, and drop in 50ml of isobutyl ketone Octane was crystallized, and the temperature was raised to 100° C. to obtain crystal form A. The sodium content was 95% to 110% as detected by atomic method.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com