Measuring method of content of arsenic in water body
A determination method and content technology, which is applied in the direction of color/spectral characteristic measurement, etc., can solve the problems of residual ozone, gaseous arsine poisonous, and carcinogenic substances, etc., and achieve the effect of streamlining the process, avoiding arsine, and simple test methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] A method for measuring arsenic content in water, specifically comprising the following steps:
[0016] 1. Prepare arsenic ion standard solution. Take 0.132g of arsenic trioxide, add water and 10mL of 0.01M sodium hydroxide solution, heat to dissolve, and finally dilute to volume in a 1000mL volumetric flask. At this time, the arsenic content in the standard solution was 0.01 mg / mL.
[0017] 2. Draw a standard curve. Take six 50mL volumetric flasks and add arsenic standard solution 0.00 / 2.00 / 4.00 / 6.00 / 8.00 / 10.00mL in turn, make 50mL to a constant volume, add 2mL of 0.01M ammonium molybdate and shake well, add 1mL of ascorbic acid and shake well, take 2mL, the wavelength is Absorbance was measured at 620 nm.
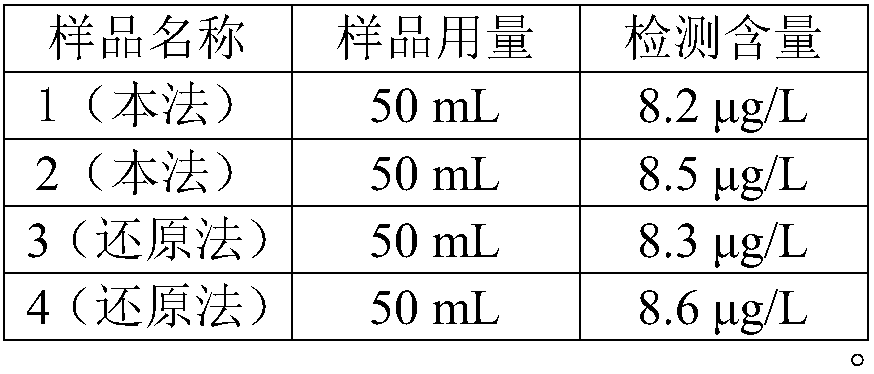
[0018] 3. Take 4 parts of 50mL hot spring water samples, centrifuge to get the supernatant for detection.
[0019] 4. Take two samples above and add 0.5g manganese dioxide to heat to completely convert trivalent arsenic into pentavalent arsenic for detection by ...
Embodiment 2
[0024] The steps of the assay method for arsenic content in a water body described in this example are the same as in Example 1, and the different technical parameters are: add 2.5mL of 0.01M ammonium molybdate in the second step and shake well, add Ascorbic acid 1.5mL shake well.
Embodiment 3
[0026] Each step of the assay method of arsenic content in a water body described in this embodiment is all the same as in Example 1, and the different technical parameters are: add 3mL of ammonium molybdate of 0.01M in the second step and shake well, add ascorbic acid 2mL and shake well.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com