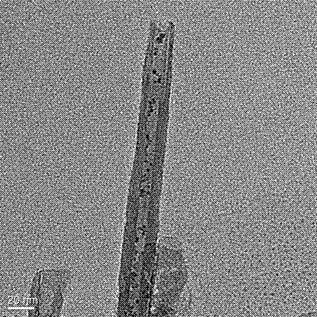
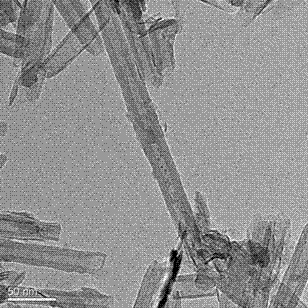
Preparation method of Rh@CuSiO3 core-sheath structured catalyst
A technology of catalysts and hydrogenation catalysts, applied in catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of poor thermal stability and crystal grain migration of active components Rh, and achieve large gas flow Quantity, solve the effect of grain migration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1.5 g of Cu(NO 3 ) 2 •6H 2 O was dissolved in water, then 25% ammonia water was added to make the solution precipitate and then dissolved again, and then ethanol was added to stir. After 10 minutes, the previously prepared 0.5mol / L NaSiO 3 •9H 2 Add 10mL of O solution into the solution to form a blue precipitate, stir for 180min, then transfer to a 150mL hydrothermal kettle, move to an oven for 12-36 hours under 473K hydrothermal treatment, then take it out for cooling, filter, wash, dry, and roast. Vested CuPNTs. Then 0.032g solid RhCl 3 Add to 25ml absolute ethanol and sonicate to aid in dissolution. RhCl 3 After all the solids are dissolved, pour 0.5 g of CuPNTs weighed in advance, and continue to sonicate. After all the CuPNTs are dissolved, sonicate for another 20 min and take it out of the ultrasonic cleaner. Add 25ml of deionized water, stir and let it stand. After layering, divide the lower layer solution into several watch glasses, dry at 80°C, and bake ...
Embodiment 2
[0027] In this example, on the basis of Example 1, the loading amount of the loaded active component is changed.
[0028] 1.5 g of Cu(NO 3 ) 2 •6H 2 O was dissolved in water, then 25% ammonia water was added to make the solution precipitate and then dissolved again, and then ethanol was added to stir. After 10 minutes, the previously prepared 0.5mol / L NaSiO 3 •9H 2 Add 10mL of O solution into the solution to form a blue precipitate, stir for 180min, then transfer to a 150mL hydrothermal kettle, move to an oven for 12-36 hours under 473K hydrothermal treatment, then take it out for cooling, filter, wash, dry, and roast. Vested CuPNTs. Then 0.077g solid RhCl 3 Add to 25ml absolute ethanol and sonicate to aid in dissolution. RhCl 3 After the solids are completely dissolved, pour 0.5g of CuPNTs weighed in advance, and continue to sonicate. After the CuPNTs are completely dissolved, sonicate for another 20 minutes and take it out of the ultrasonic cleaner. Add 25ml of deion...
Embodiment 3
[0030] In this example, on the basis of Example 1, the loading amount of the loaded active component is changed.
[0031] 1.5 g of Cu(NO 3 ) 2 •6H 2 O was dissolved in water, then 25% ammonia water was added to make the solution precipitate and then dissolved again, and then ethanol was added to stir. After 10 minutes, the previously prepared 0.5mol / L NaSiO 3 •9H 2 Add 10mL of O solution into the solution to form a blue precipitate, stir for 180min, then transfer to a 150mL hydrothermal kettle, move to an oven for 12-36 hours under 473K hydrothermal treatment, then take it out for cooling, filter, wash, dry, and roast. Vested CuPNTs. Then 0.113g solid RhCl 3 Add to 25ml absolute ethanol, sonicate to help dissolve. RhCl 3After the solids are completely dissolved, pour 0.5g of CuPNTs weighed in advance, and continue to sonicate. After the CuPNTs are completely dissolved, sonicate for another 20 minutes and take them out of the ultrasonic cleaner. Add 25ml of deionized wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com