High-purity fipronil preparation method
A fipronil and high-purity technology is applied in the field of preparation of high-purity fipronil, which can solve the problems such as the need to improve the overall yield and the long operation steps, and achieve the effects of conventional equipment, easy operation, and high product yield and purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
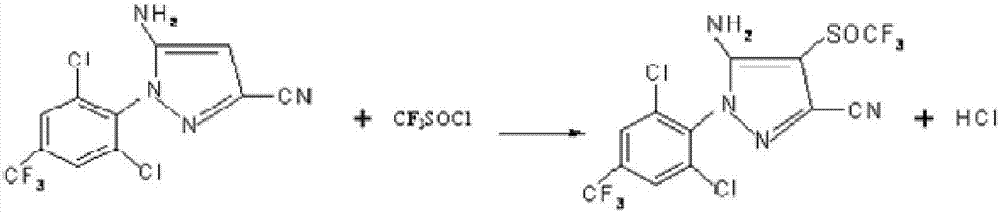
[0026] Under the micro-vacuum condition with a vacuum degree of -0.1Mpa, 120kg of trifluoromethylthionyl chloride, 450kg of 5-amino-3-cyano-1-(2,6-dichloro-4- Trifluoromethylphenyl) pyrazole, 10kg catalyst trimethylbenzyl ammonium chloride and 1900kg solvent ethylene dichloride, after reacting at 40 ℃ for 1.5 hours, add 120kg trifluoromethylthionyl chloride again, after adding After continuing the insulation reaction for 10 hours, the reaction solution was added to 1000kg of water, allowed to stand for stratification, and centrifuged to filter, and the filtrate was distilled to reclaim ethylene dichloride to recover and apply mechanically to obtain 560kg of filter cake A, then add 560kg of butyl acetate and 2240kg of toluene, and heat up to 85°C, stir until completely dissolved, then lower the temperature to 0°C, keep it warm for 3 hours, then centrifuge, and dry the filter cake B to obtain 450kg of fipronil. The purity of fipronil is determined to be 98%, and the yield is 88....
Embodiment 2
[0028] Under the micro-vacuum condition with a vacuum degree of -0.08Mpa, 110kg of trifluoromethylthionyl chloride, 470kg of 5-amino-3-cyano-1-(2,6-dichloro-4- Trifluoromethylphenyl) pyrazole, 10kg catalyst trimethylbenzyl ammonium chloride and 1500kg solvent ethylene dichloride, after reacting at 35 ℃ for 2 hours, add 110kg trifluoromethylthionyl chloride again, after adding After continuing the insulation reaction for 9 hours, the reaction solution was added to 1000kg of water, allowed to stand for stratification, centrifuged, and the filtrate was distilled to recover dichloroethane and used mechanically to obtain 548kg of filter cake A. 88°C, stir until completely dissolved, then lower the temperature to 2°C, keep it warm for 2 hours, then centrifuge, and dry the filter cake B to obtain 443kg of fipronil. The purity of fipronil is determined to be 97.6%, and the yield is 90.3%.
Embodiment 3
[0030] Under the micro-vacuum condition with a vacuum degree of -0.05Mpa, 125kg of trifluoromethylthionyl chloride, 430kg of 5-amino-3-cyano-1-(2,6-dichloro-4- Trifluoromethylphenyl) pyrazole, 10kg catalyst trimethylbenzyl ammonium chloride and 2000kg solvent ethylene dichloride, after reacting at 45 ℃ for 1 hour, add 125kg trifluoromethylthionyl chloride again, after adding After continuing the heat preservation reaction for 12 hours, add the reaction solution into 1000kg of water, let it stand for stratification, centrifugally filter, and distill the filtrate to recover dichloroethane and apply it mechanically to obtain 578kg of filter cake A, then add 867kg of n-hexane and 2601kg of toluene, and heat up to 90°C , stirred until completely dissolved, lowered the temperature to 5°C, kept the temperature for 5 hours and then centrifuged, and dried the filter cake B to obtain 458kg of fipronil. The purity of fipronil was determined to be 98.2%, and the yield was 89.7%. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com