Cyanuric fluoride preparation method
A technology of cyanuric fluoride and fluorine reaction, which is applied in the direction of organic chemistry, can solve the problems of strong corrosion of equipment, low product yield, high energy consumption of solvent recovery, etc., and achieve strong corrosion of equipment, high energy consumption, Simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
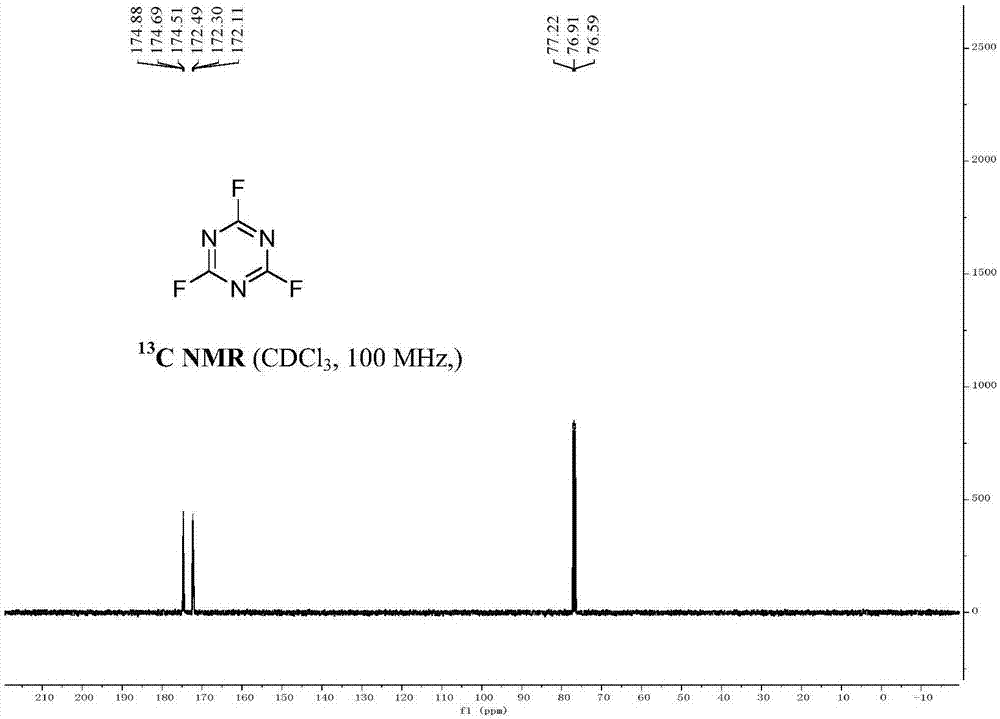
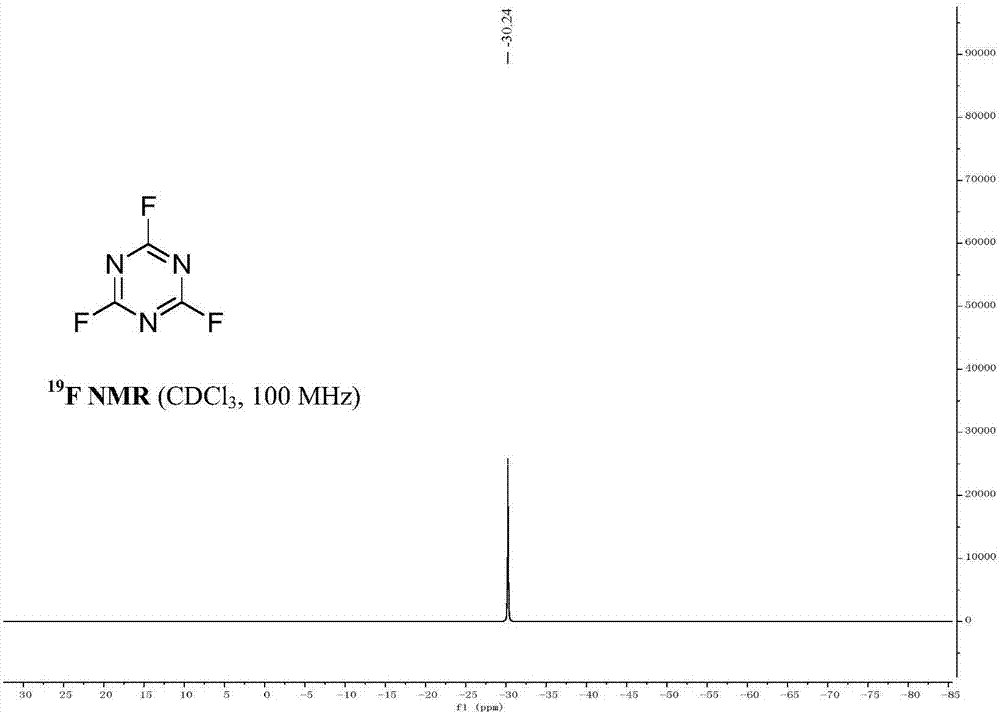
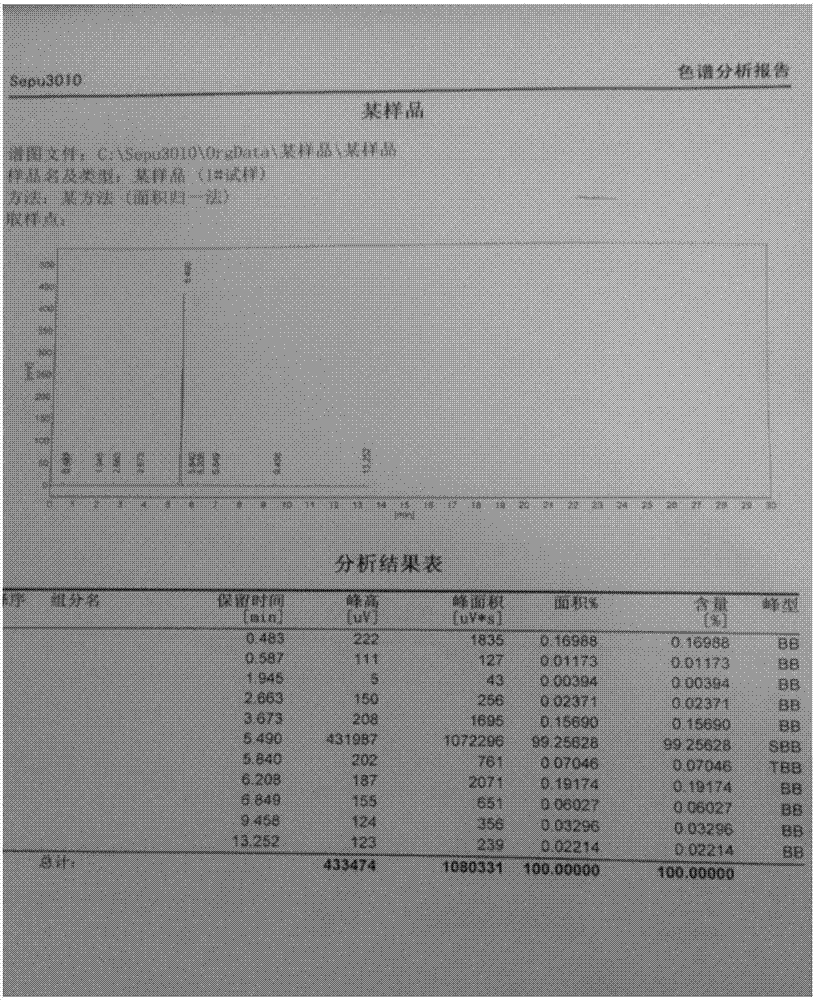
[0026] In a dry reaction vessel, add 1,3,5-trichloro-s-triazine (185g, 1mol) and tetrabutylammonium fluoride (861g, 3.3mol), anhydrous dimethyl sulfoxide (390g, 5.0mol) . The reaction was stirred at 50°C for 6 hours, (TCL followed the reaction). The reaction device was changed into a distillation device, vacuum distillation, the reduced pressure was 0.012 MPa, and 124.2 grams of colorless liquid with a boiling point of about 70° C. was collected. The content of the product measured by GC was 99.2%, and the yield was 92%. Under low temperature conditions, cyanuric fluoride is stored in containers made of polytetrafluoroethylene.
Embodiment 2
[0030] In a dry reaction vessel, add 1,3,5-trichloro-s-triazine (370g, 2mol) and tetrabutylammonium fluoride (1.722kg, 6.6mol), anhydrous dimethyl sulfoxide (780g, 10.0mol ), stirred and reacted at 60°C for 6 hours. (TCL tracking response). The reaction device was changed into a distillation device, vacuum distillation, the reduced pressure was 0.013 MPa, and 257 grams of colorless liquid with a boiling point of about 70° C. was collected. The content of the product measured by GC was 99.0%, and the yield was 95%. Under low temperature conditions, cyanuric fluoride is stored in containers made of polytetrafluoroethylene.
Embodiment 3
[0034] In a dry reaction vessel, add 1,3,5-trichloro-s-triazine (278g, 1.5mol) and tetrabutylammonium fluoride (1.57kg, 6.0mol), anhydrous dimethyl sulfoxide (702g, 9.0 mol), the reaction was stirred at 60°C for 6 hours. (TCL tracking response). The reaction device was changed into a distillation device, vacuum distillation, the reduced pressure was 0.013MPa, and 188 grams of colorless liquid with a boiling point of about 70° C. was collected. The content of the product measured by GC was 99.0%, and the yield was 93%. Under low temperature conditions, cyanuric fluoride is stored in containers made of polytetrafluoroethylene.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com