Medical application of catalpol and radix astragali extracting solution
A technology of astragalus extract and catalpol, applied in the field of medicine, can solve the problems of uncurable ALS, liver and kidney function damage, and difficult treatment, and achieve the effect of preventing or delaying motor neuron disease, delaying the onset time, and having a good market prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The medicine for preventing or delaying motor neuron disease, nerve injury or / and degenerative disease and alleviating ALS, its raw material and preparation method are as follows:
[0024] Preparation method of Astragalus water extract: take Astragalus, add water equivalent to 8-10 times the weight of Astragalus and soak for 45 minutes, then heat and reflux extraction twice, each time for 1 hour, combine the extracts, and concentrate the extracts to 4 times the weight of Astragalus. times the concentrated solution to obtain the Radix Astragali water extract.
[0025] The preparation method of rehmannia glutinosa water extract: take the rehmannia glutinosa of the formula, add water equivalent to 8-10 times the weight of rehmannia glutinosa and soak for 45 minutes, then heat and reflux for extraction twice, each time for 1 hour, combine the extracts, and concentrate the extracts to the equivalent The concentrated solution of 4 times the weight of Rehmannia glutinosa is ob...
Embodiment 2
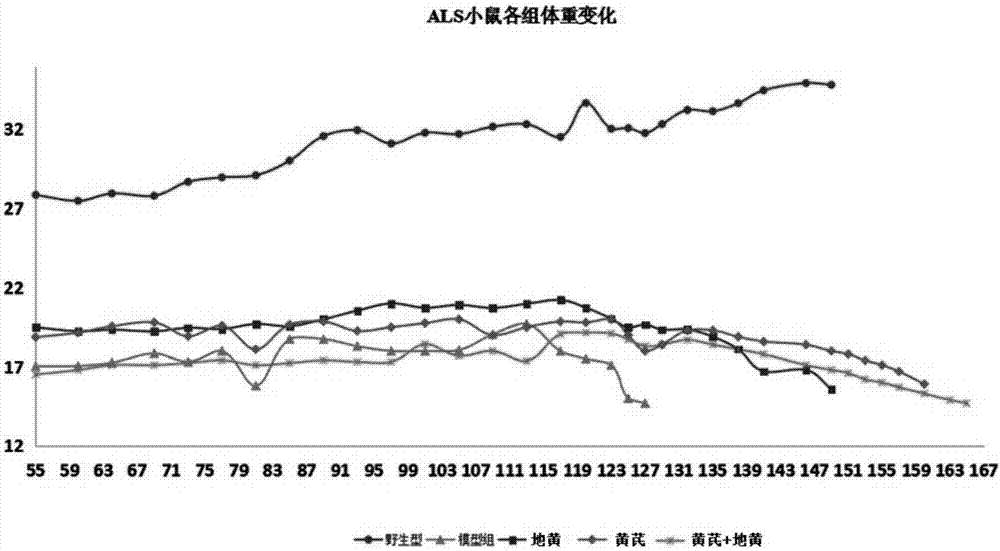
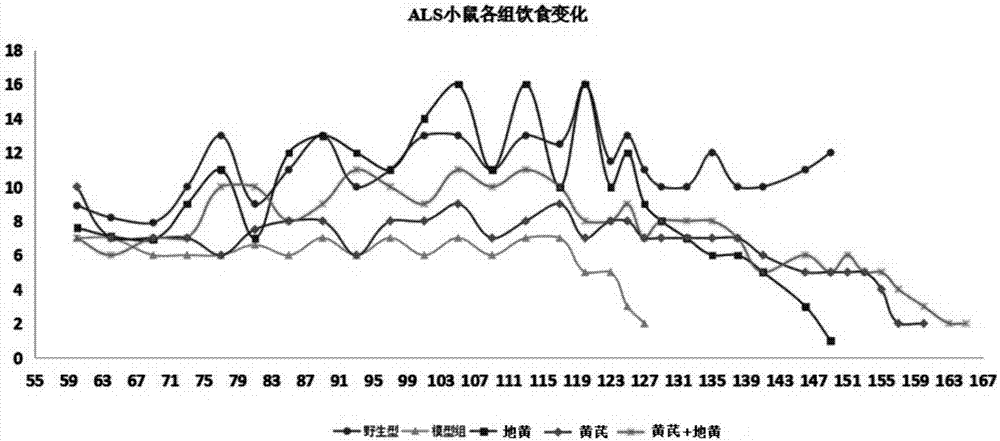
[0032] Pharmacodynamic investigation and evaluation of Astragalus root and Rehmannia glutinosa extracts in the treatment of amyotrophic lateral sclerosis (ALS), the specific methods are as follows: purchase 30 hSOD1-G93A transgenic mice and 6 wild type B6SJLF1 / J+ / + mice, The wild-type B6SJLF1 / J+ / + mice were used as the normal control NC group (n=6), and the hSOD1-G93A transgenic mice were randomly divided into 5 groups, which were named: ALS control group (n=6), positive drugs Luzole treatment group (Riluzole, n=6), Dihuang treatment group (catalpol 10mg / kg, n=6), Huangqi injection group (12g / kg, n=6), catalpol+Dihuang injection group (n =6). The specific grouping, dosage and method of administration are shown in Table 1:
[0033] Table 1. The specific grouping of experimental mice and the dosage and administration method
[0034]
[0035] Then evaluate their water intake and food intake every 4 days, and evaluate their food intake and body weight changes. The body weigh...
Embodiment 3
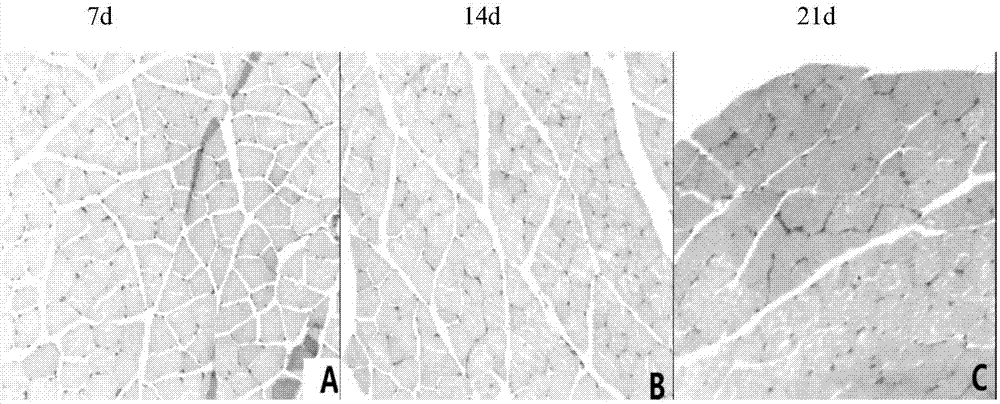
[0061] Establishment of animal model of sciatic nerve injury: the animals were anesthetized by intraperitoneal injection of 3.5% chloral hydrate at a dose of 0.1ml / 10g, after binding, routine disinfection and skin preparation. Cut open the skin with a scalpel blade and make a median longitudinal incision at the posterior part of the right femur, and suddenly separate the muscles to expose the sciatic nerve at the lower border of the piriformis muscle. The hemostat clamps the sciatic nerve to the third tooth, four times in total, 15 seconds each time. After completion, the nerve was returned and the skin was sutured. In the sham operation group, only the sciatic nerve was exposed without clamping, and then the wound was sutured.
[0062] Animal grouping and administration: 60 mice successfully modeled were randomly divided into five groups: sham operation group, model group, rehmannia glutinosa alcohol extract group (60g / kg), astragalus alcohol extract group (60g / kg, concentra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com