Application of diphenhydramine hydrochloride in preparation of medicine for treating experimental autoimmune encephalomyelitis
A diphenhydramine hydrochloride, autoimmune technology, applied in the field of medicine, can solve the problems of incomplete cure of MS, poor prognosis of multiple sclerosis, etc., and achieve the effect of inhibiting the incidence of inflammation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
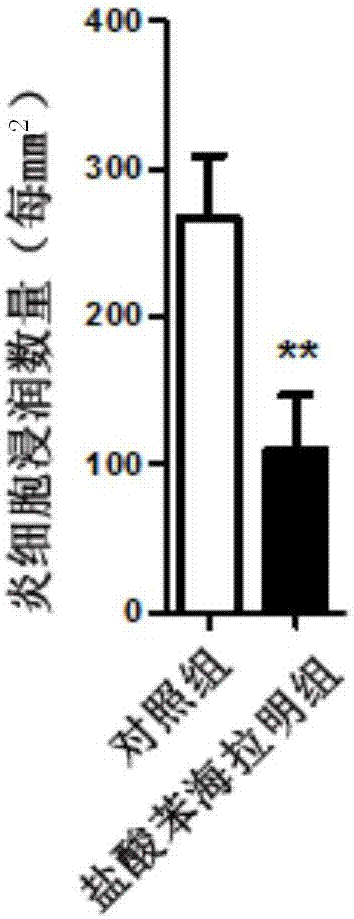
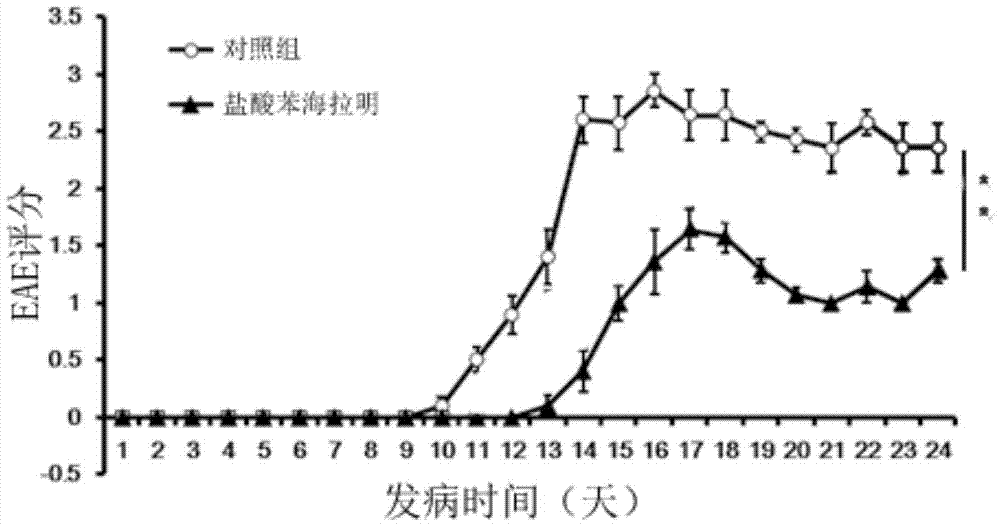
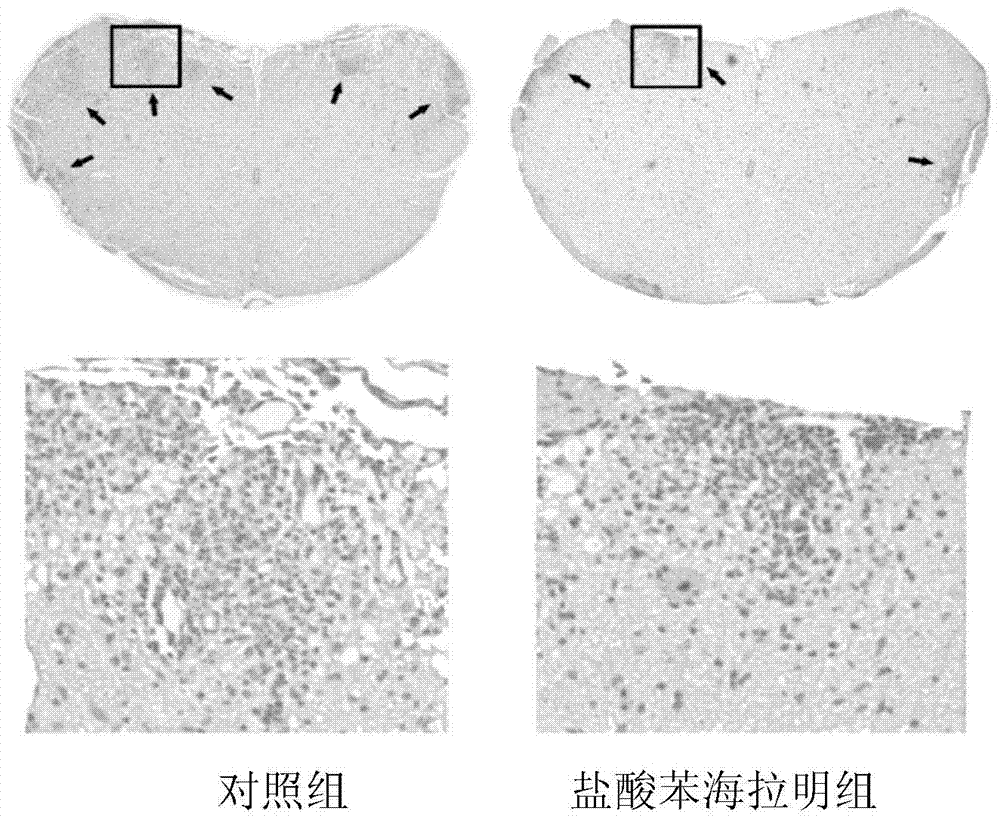
[0035] Embodiment 1: the effect of diphenhydramine hydrochloride in experimental autoimmune encephalomyelitis (EAE)
[0036] Materials and methods
[0037] 1. Experimental animals
[0038] C57BL / 6J mice (female, 8-12 weeks old) were purchased from Nanjing Institute of Biomedicine and Shanghai Slack Experimental Animal Co., Ltd. All animal experiments were approved by the Animal Ethics Committee of the Second Military Medical University.
[0039] 2. EAE modeling and in vivo diphenhydramine hydrochloride intervention
[0040] 1) Preparation of complete Freund's adjuvant: Dissolve tuberculin Mtb (purchased from Difco, USA) in incomplete Freund's adjuvant CFA (purchased from sigma), add CFA while grinding with a ceramic mortar, and finally dissolve The concentration is 4mg / ml;
[0041] 2) MOG 35-55 powder (myelin oligodendrocyte glycoprotein 35-55 , purchased from Jill Biochemical Company) was dissolved in sterile PBS, and the final dissolved concentration was 2mg / ml;
[00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com