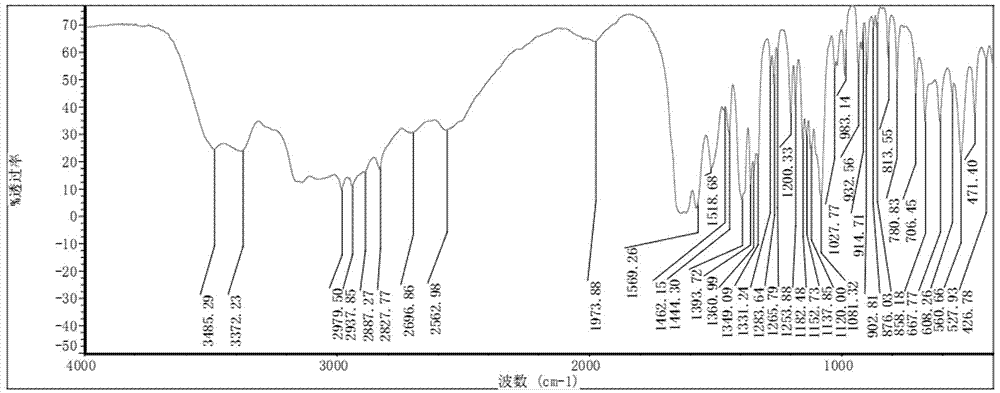
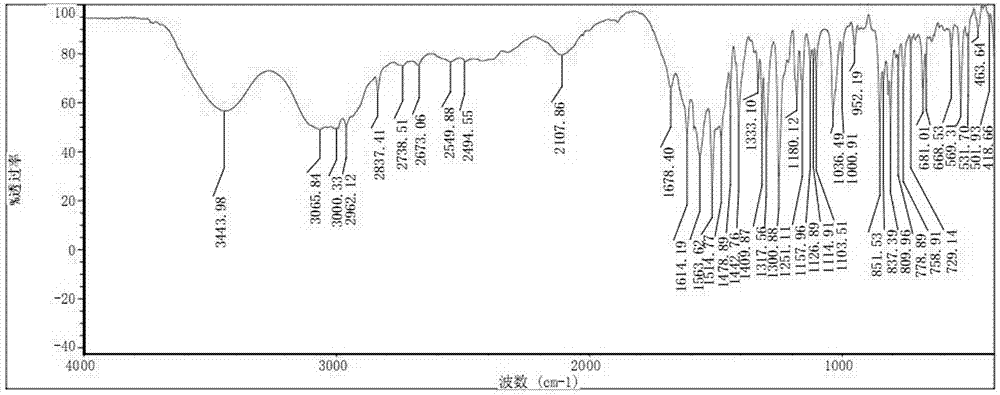
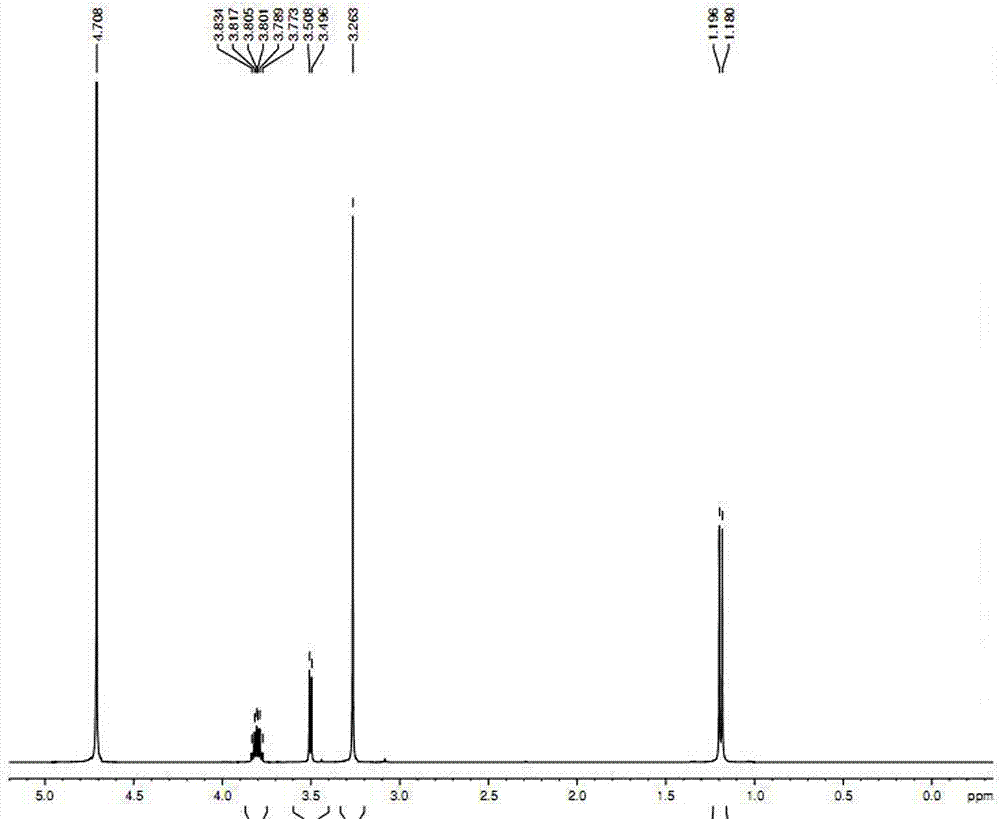
Preparation method of O-methyl-threonine/tyrosine
A technology of tyrosine and threonine, which is applied in the field of preparation of threonine/tyrosine derivatives, can solve the problems of dangerous raw materials, long steps, unfriendly environment, etc., and achieve the effect of reliable operability and short reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Add N-tert-butoxycarbonyl-L-threonine into acetone, after all dissolve, keep the reaction system at 20-35°C, add 8 equivalents of sodium hydroxide in batches, stir for 2 hours after the addition, keep the reaction system at 0 -20°C, add 4 equivalents of dimethyl sulfate dropwise, react overnight at room temperature (20-30°C) after addition, add water to the reaction system, evaporate acetone at 40-50°C, after removing acetone, add Ethyl acetate and crushed ice, acidified with solid citric acid to pH=2-3, separated layers, discarded the water phase, washed the oil phase with saturated brine three times, dried over sodium sulfate for 2 hours, filtered to remove sodium sulfate, evaporated Part of ethyl acetate was added with petroleum ether and stirred for crystallization to obtain N-tert-butoxycarbonyl-O-methyl-L-threonine with a yield of 61%. Dissolve N-tert-butoxycarbonyl-O-methyl-L-threonine in acetone, keep at 10-25°C, add 4 equivalents of p-toluenesulfonic acid in ba...
Embodiment 2
[0018] Add N-tert-butoxycarbonyl-D-threonine into acetone, after all dissolve, keep the reaction system at 20-35°C, add 8 equivalents of sodium hydroxide in batches, stir for 2 hours after the addition, keep the reaction system at 0 -20°C, add 4 equivalents of dimethyl sulfate dropwise, react overnight at room temperature (20-30°C) after addition, add water to the reaction system, evaporate acetone at 40-50°C, after removing acetone, add Ethyl acetate and crushed ice, acidified with solid citric acid to pH=2-3, separated layers, discarded the water phase, washed the oil phase with saturated brine three times, dried over sodium sulfate for 2 hours, filtered to remove sodium sulfate, evaporated Part of ethyl acetate was added with petroleum ether and stirred for crystallization to obtain N-tert-butoxycarbonyl-O-methyl-D-threonine with a yield of 58%. Dissolve N-tert-butoxycarbonyl-O-methyl-D-threonine in acetone, keep at 10-25°C, add an equivalent amount of trifluoroacetic acid ...
Embodiment 3
[0020] Add N-tert-butoxycarbonyl-DL-threonine into acetone, after all dissolved, keep the reaction system at 20-35°C, add 8 equivalents of sodium hydroxide in batches, stir for 2 hours after the addition, keep the reaction system at 0 -20°C, add 4 equivalents of dimethyl sulfate dropwise, react overnight at room temperature (20-30°C) after addition, add water to the reaction system, evaporate acetone at 40-50°C, after removing acetone, add Ethyl acetate and crushed ice, acidified with solid citric acid to pH=2-3, separated layers, discarded the water phase, washed the oil phase with saturated brine three times, dried over sodium sulfate for 2 hours, filtered to remove sodium sulfate, evaporated Part of ethyl acetate was added with petroleum ether and stirred to crystallize to obtain N-tert-butoxycarbonyl-O-methyl-DL-threonine with a yield of 63%. N-tert-butoxycarbonyl-O-methyl-DL-threonine was dissolved in acetone, kept at 10-25°C, added 4 equivalents of p-toluenesulfonic acid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com