Alkaline protease BmP mutants with improved thermal stability and genes and application thereof
A technology of thermal stability and mutant, applied in application, genetic engineering, plant genetic improvement, etc., can solve the problems of poor thermal stability of BmP, easy decomposition and inactivation, and limited industrial application, and achieve the effect of improving stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1, the cloning of bacillus mojavensis (Bacillus mojavensis) alkaline protease BmP gene
[0048] The target gene was directly synthesized according to the sequence of the alkaline protease gene of Bacillus mohaiwei (Genebank: AY665611.1). Two primers (R: 5'-ATCGGGATCCGCTCAACCGGCGAAAAATGTT-3' and F: 5'-TCTAGCGGCCGC TTATTGAGCGGCAGCTTCGAC-3') were designed according to the synthesized target gene to amplify the BmP gene of Bacillus mohaiwei alkaline protease. The amplified PCR product was purified and recovered, and connected to the expression vector phyP 43 L, get the expression vector phyP 43 L-BmP.
Embodiment 2
[0049] Embodiment 2, site-directed saturation mutation
[0050] The effect of position 193 and position 288 on the thermostability of alkaline protease BmP was studied by saturation mutagenesis. The process of site-specific saturation mutation is as follows: To construct a good phyP 43 L-BmP was used as a template, and the corresponding mutant primers were used for PCR amplification; the amplified PCR product was subjected to agarose electrophoresis, and the PCR product was purified and recovered. Decompose the original plasmid with the restriction endonuclease DpnI, transfer the decomposed product into E. coli Top10 by heat shock method, verify the recombinant transformant by bacterial liquid PCR, extract and verify the correct transformant plasmid for sequencing, so as to determine the corresponding mutants. The mutants with correct sequencing were transformed into Bacillus subtilis WB600 by electrotransformation.
[0051] The screening of recombinant transformants is as ...
Embodiment 3
[0054] Embodiment 3, combinatorial mutation
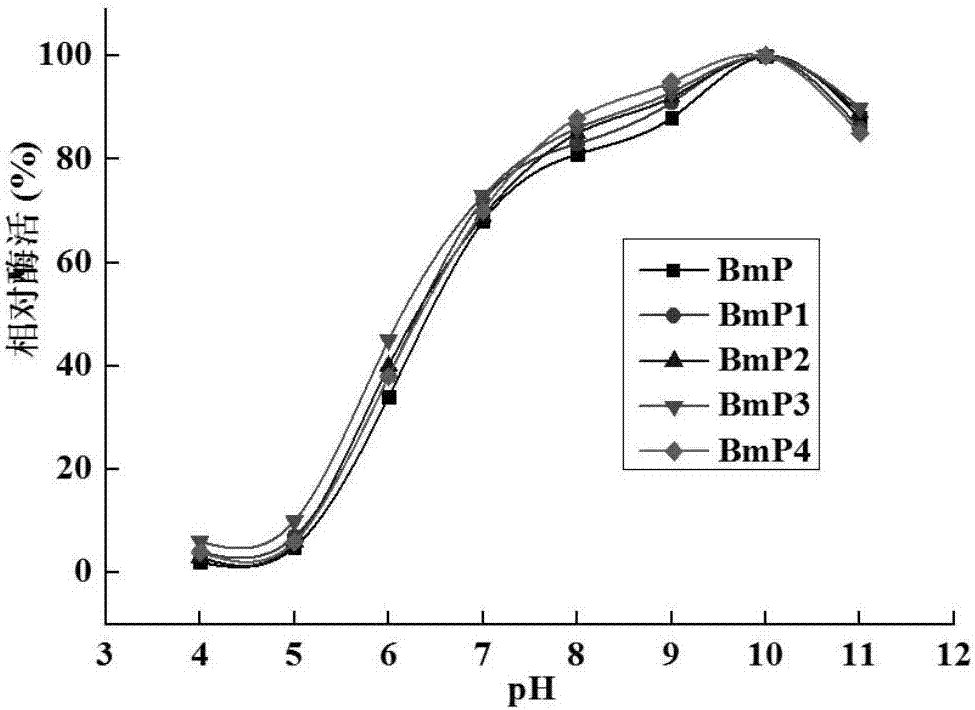
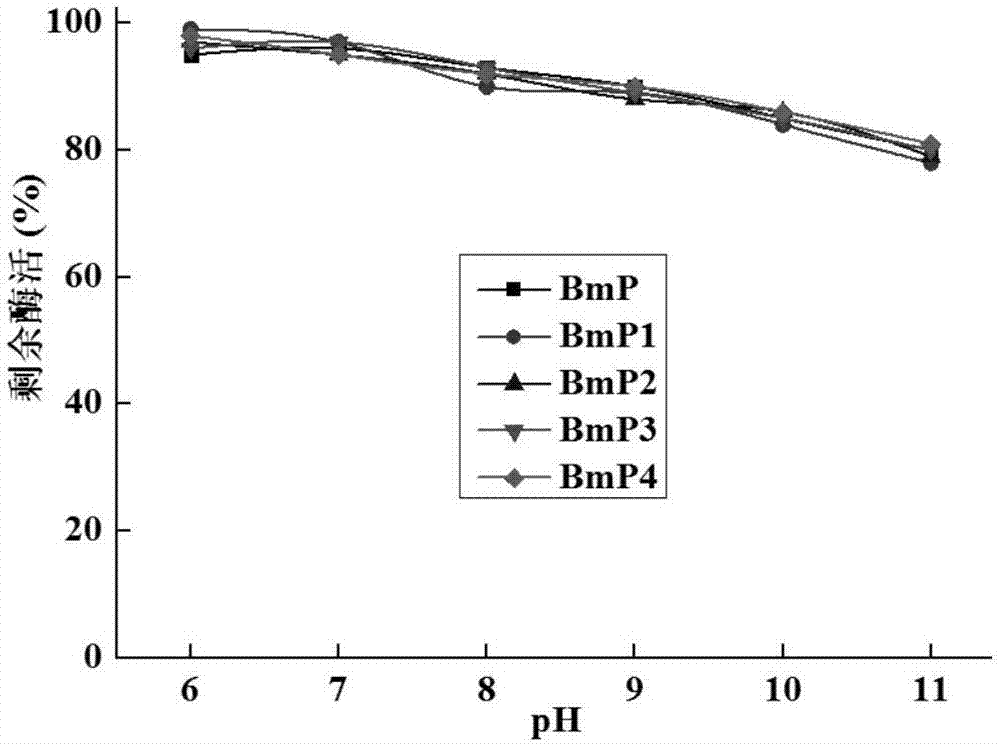
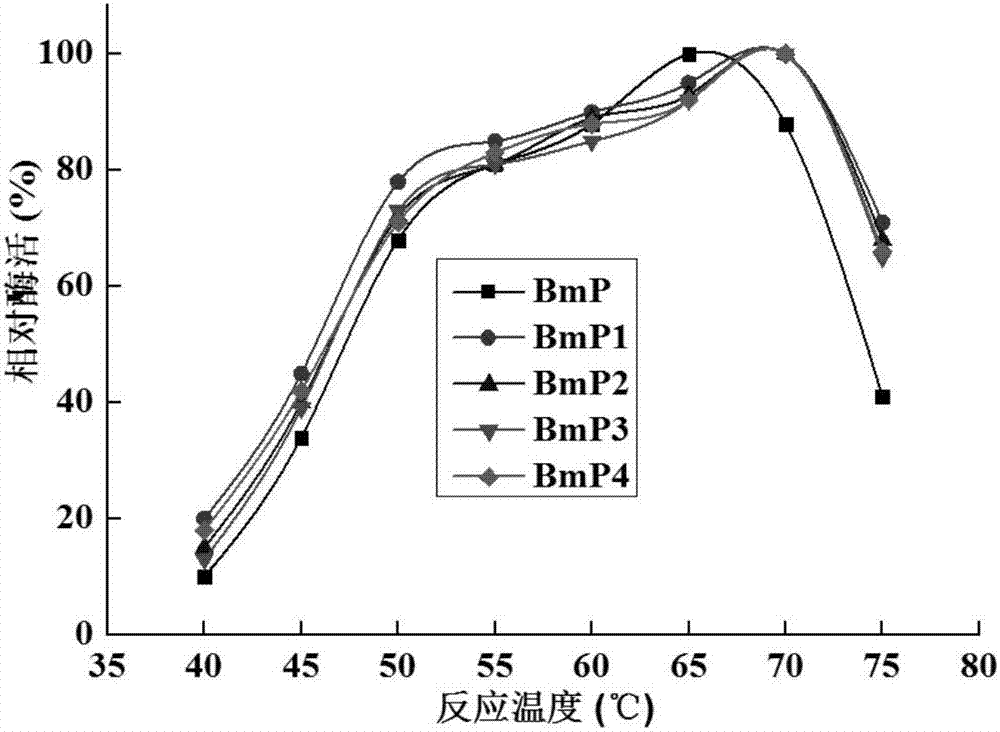
[0055] Combined mutations were carried out on the basis of single point mutations, and four combined mutations were finally obtained through experiments, named BmP1, BmP2, BmP3, and BmP4, respectively. The mutation sites contained in BmP1 are S193K and S288M; the mutation sites contained in BmP2 are S193L and S288M; the mutation sites contained in BmP3 are S193L and S288K; the mutation sites contained in BmP3 are S193K and S288K.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com