Polyhydroxy pregnane compound and preparation method thereof and application of polyhydroxy pregnane compound in preparation of anticomplementary drugs
A polyhydroxypregnane, compound technology, applied in the field of traditional Chinese medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
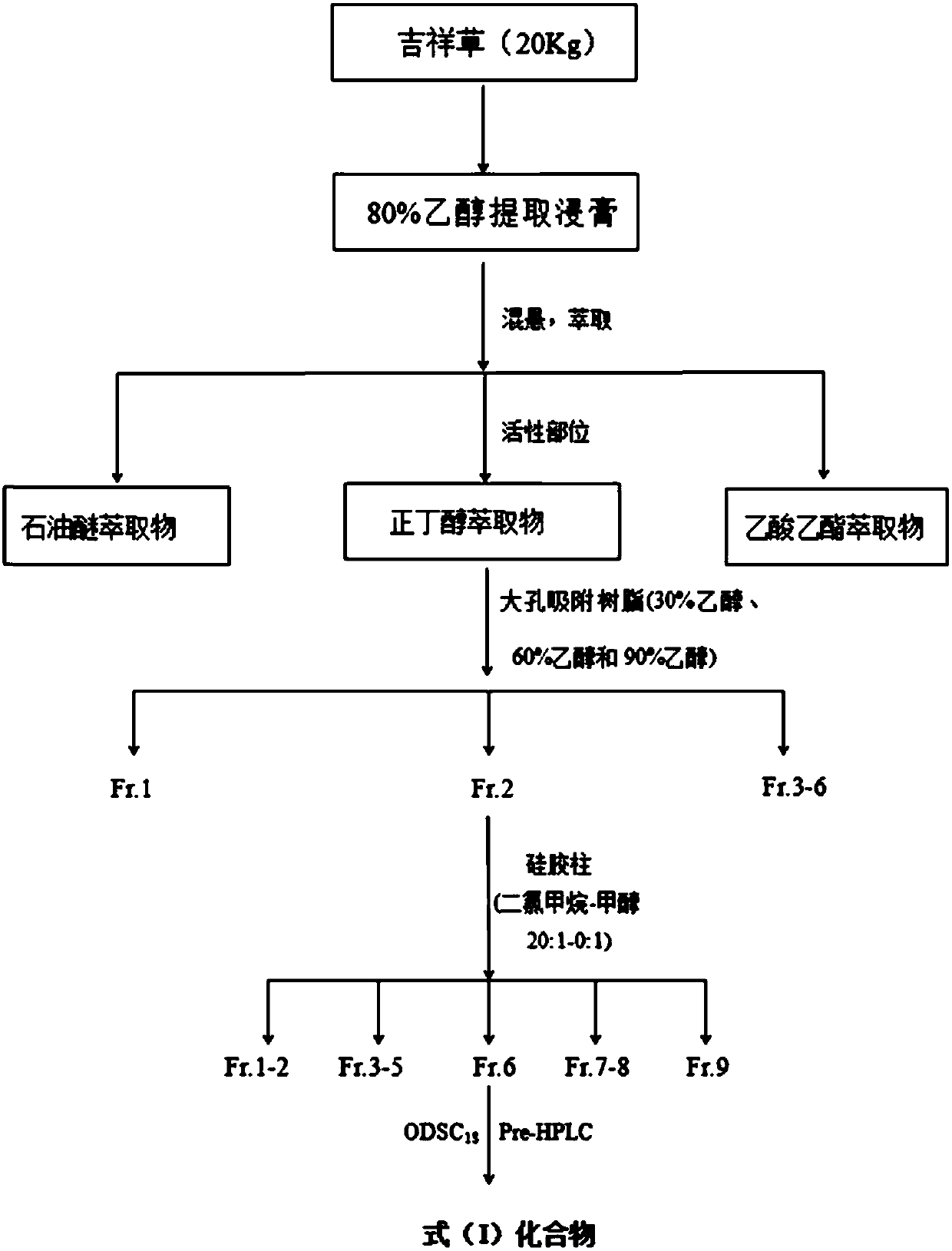
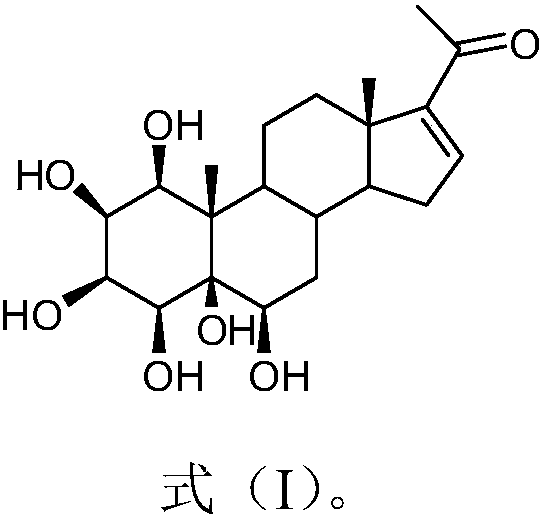
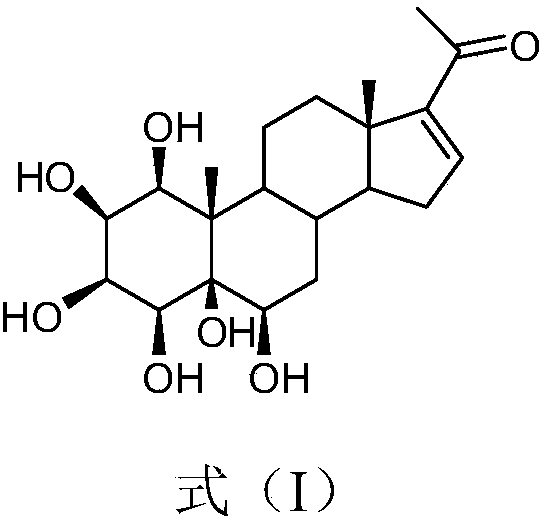
[0021] The extraction and separation method of embodiment 1. formula (I) compound
[0022] Take 20kg (dry) of the whole auspicious grass purchased from the market, heat and reflux with 80% ethanol and extract 3 times, the extraction time is 2h, 2h, 1h respectively, combine the three extracts, concentrate under reduced pressure until there is no alcohol smell (about 15L), and then It was extracted with petroleum ether, ethyl acetate and n-butanol, and concentrated to dryness respectively, and 780 g of n-butanol extract was obtained. Take n-butanol part extract and separate with macroporous adsorption resin HP20, elute sequentially with ethanol: water (30%, 60%, 95% ethanol) to get 30% ethanol elution fraction Fr.1 (188g), 60% ethanol Ethanol-eluted fraction Fr.2 (241 g), 95% ethanol-eluted fraction Fr.3 (79 g). Fraction Fr.2 was separated by silica gel column chromatography and eluted with dichloromethane-methanol (20:1-0:1) gradient to obtain 6 fractions Fr.4-9. Fraction Fr....
Embodiment 2
[0024] Example 2. In vitro anti-complement classical pathway test of the compound of formula (I)
[0025]This example reflects the magnitude of the effect of the compound of formula (I) on complement through the anti-complement activity of the classical pathway. The experimental method is as follows:
[0026] 1. The preparation method of the test product control group:
[0027] Weigh about 3mg of the test product, dissolve it with 20uL DMSO, add 780uL of barbiturate buffer, draw 400uL into the EP tube, and dilute into eight concentration gradients successively for testing. Add 200uL complement, 100uL hemolysin and sheep red blood cells each.
[0028] 2. Preparation method of complement group:
[0029] Add 200uL complement, 100uL hemolysin and sheep red blood cells to the complement group, and then add 200uL barbiturate buffer.
[0030] 3. Experimental method: Take 0.1ml of complement (guinea pig serum), add barbiturate buffer solution (BBS) to make a 1:10 solution, and dil...
Embodiment 3
[0031] Example 3. In Vitro Anti-Complement Alternative Pathway Test of Compounds of Formula (I)
[0032] This example reflects the magnitude of the effect of the compound of formula (I) on complement through the anti-complement activity of the alternative pathway. The experimental method is as follows:
[0033] 1. The preparation method of the test product control group:
[0034] Weigh about 3mg of the test product, dissolve it with 20uL DMSO, add 780uL of barbiturate buffer, draw 400uL into the EP tube, and dilute into eight concentration gradients successively for testing. Add 200uL complement, 100uL hemolysin and rabbit red blood cells each.
[0035] 2. Preparation method of complement group:
[0036] Add 200uL complement, 100uL hemolysin and rabbit red blood cells to the complement group, and then add 200uL barbiturate buffer.
[0037] 3. Experimental method: Take 0.2ml of complement (human serum), add AP diluent (barbital buffer, pH=7.4, containing 5mM Mg 2+ ,8mM EGT...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com