6,8-methano-tetrahydroquinazoline-2-amine schiff base iron ion fluorescence probe, and preparation method and application thereof
A technology of endomethylenetetrahydroquinazoline and endomethylenequinazoline, which is applied in the field of fine organic synthesis, and can solve the problems of not synthesizing nopinone pyridine Schiff base compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation method of 6,8-methanotetrahydroquinazolin-2-amine Schiff base compound is:
[0026]
[0027] Specific steps are as follows:
[0028] Preparation of 6,8-methanotetrahydroquinazolin-2-amine Schiff bases:
[0029] 2.0mmol 7,7-dimethyl-4-phenyl-5,6,7,8-tetrahydro-6,8-methanoquinazolin-2-amine, 2.4mmol pyridine-2-carbaldehyde , 2.5mmol acetic acid and 60mL ethanol were sequentially added to a three-necked flask equipped with a stirrer, a thermometer and a reflux condenser, heated to reflux for 28 hours, followed by LC-MS detection until the compound was converted again, the reaction was terminated, and the reactant was concentrated to 8mL , then cooled to room temperature, and after standing for 2 days, a pale yellow crystal 7,7-dimethyl-4-phenyl-N-(pyridine-2-methylene)-5,6,7,8-tetrahydro- 6,8-Methylenequinazolin-2-amine is compound (1), and the yield is 42%.
[0030] The product is characterized, the data are as follows:
[0031] mp:58.2-58.6℃;IR(KBr...
Embodiment 2
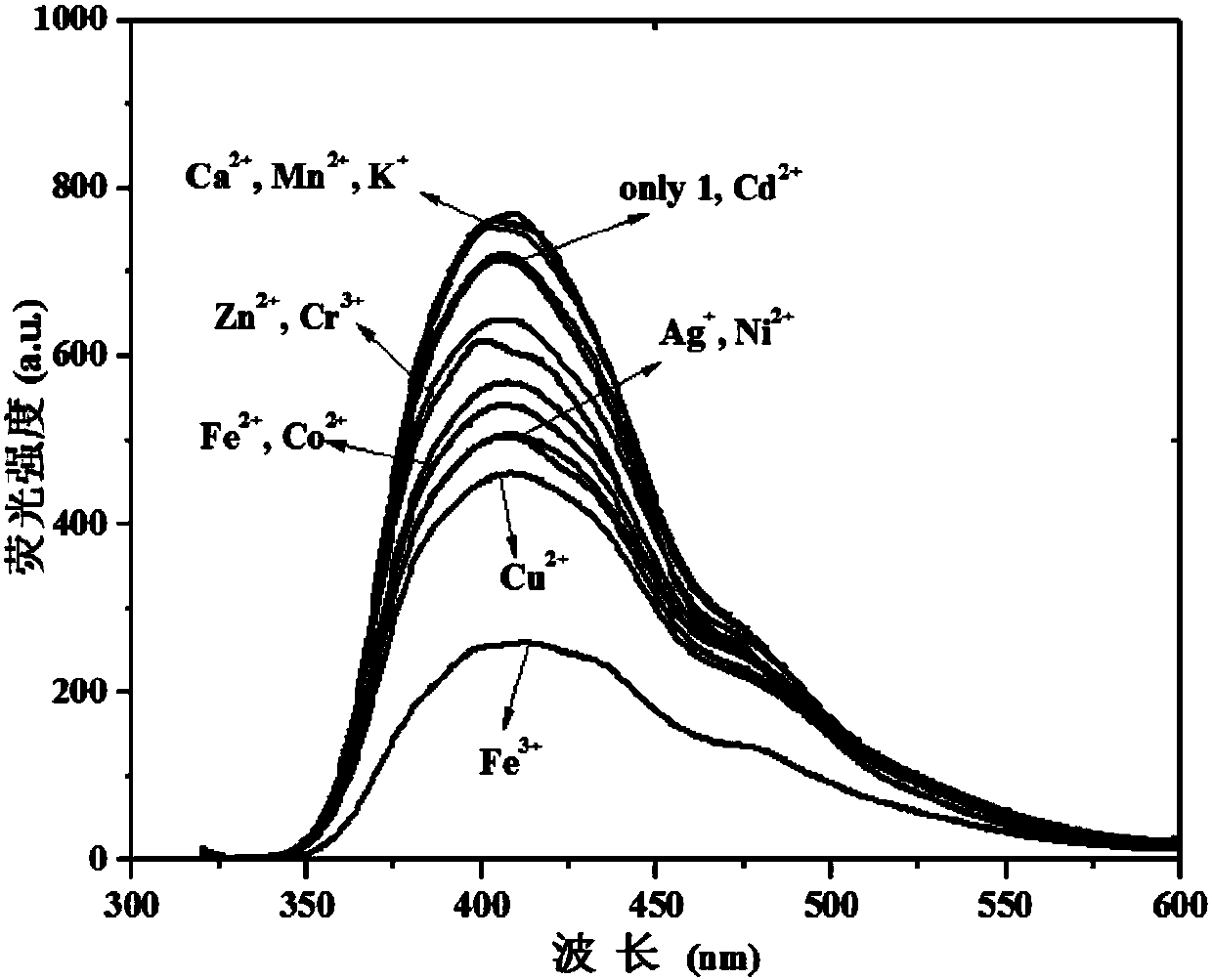
[0036] Compound (1) was prepared as 1×10 -4 The ethanol solution of M, all kinds of metal ions are also dissolved in ethanol to make a concentration of 1×10 -4 M's solution. Measure the fluorescence emission spectra of different metal ions to compound (1), such as figure 1 shown. The results show that with the addition of iron ions, the fluorescence intensity of the system is significantly reduced, and by adding the same amount of other metal ions, such as Mn 2+ ,Zn 2+ , K + , Ni 2+ , Ca 2+ ,Cd 2+ , Fe 2+ ,Cu 2+ , Ag + ,Co 2+ ,Cr 3+ According to plasma contrast observation, the fluorescence spectrum of the compound does not change significantly, which indicates that the compound can be used as a fluorescent probe for specifically recognizing iron ions.
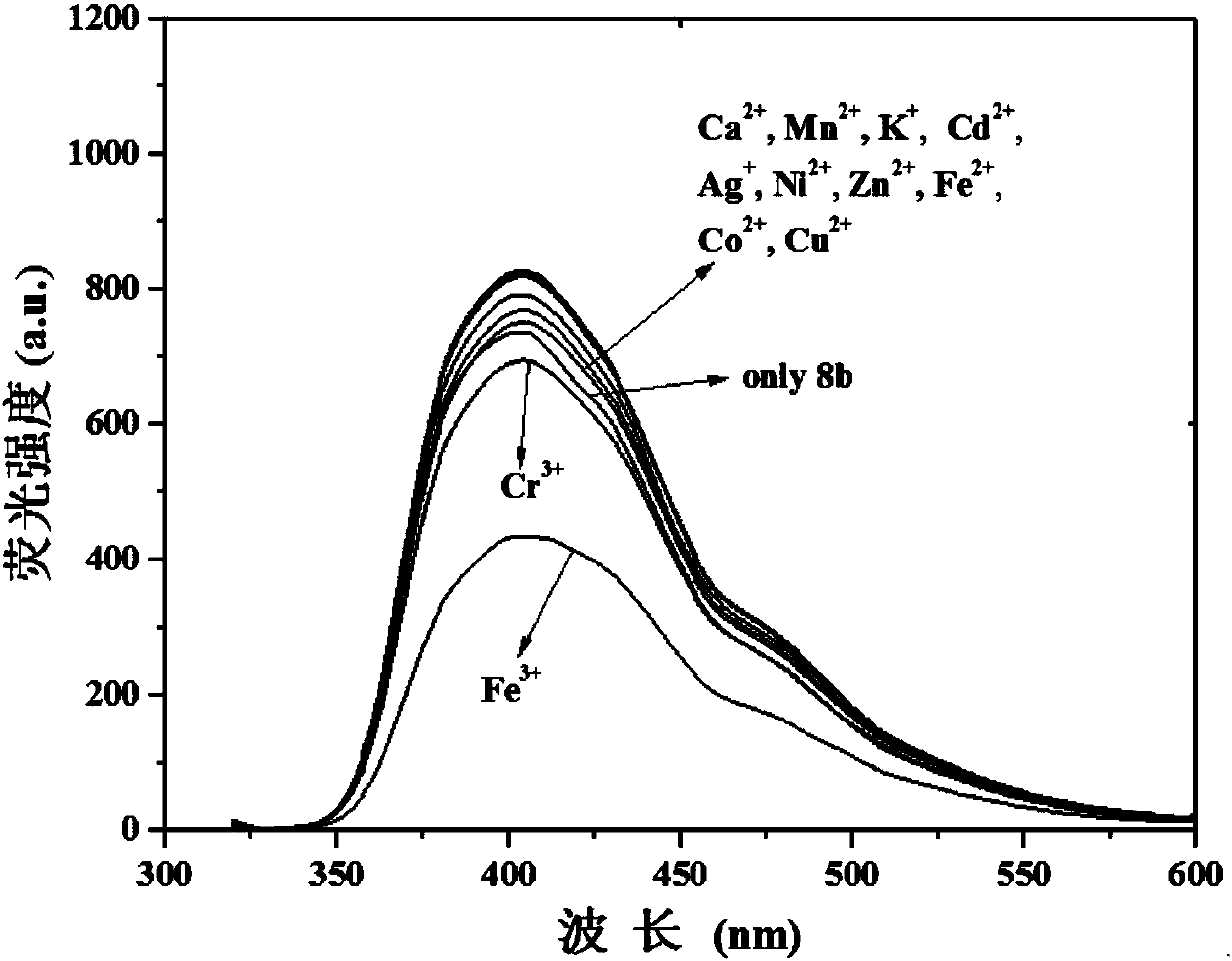
[0037] Compound (2) was prepared as 1×10 -4 The ethanol solution of M, all kinds of metal ions are also dissolved in ethanol to make a concentration of 1×10 -4 M's solution. Measure the fluorescence spectrum of...
Embodiment 3
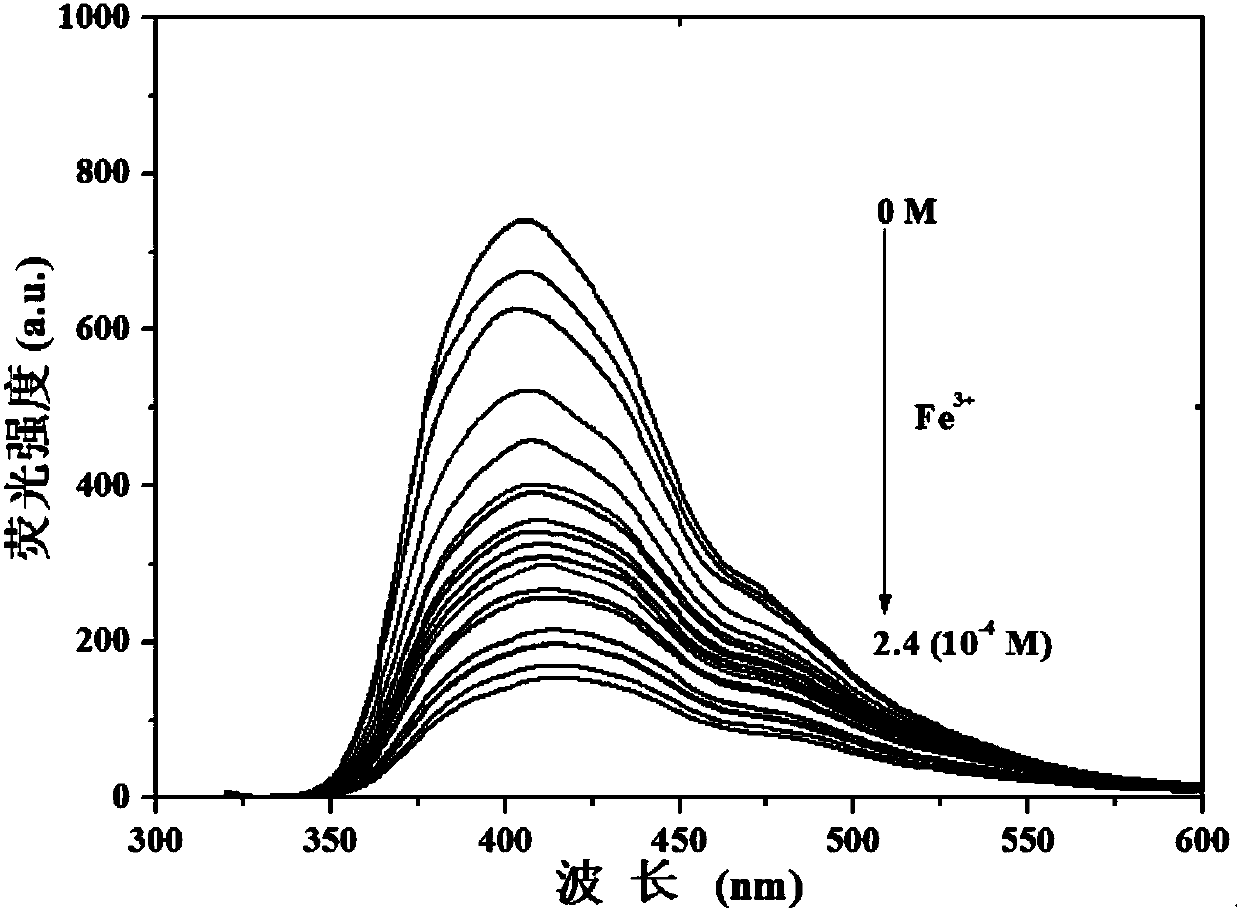
[0039] Compound (1) was prepared as 1×10 -4 M ethanol solution, dissolving iron ions in ethanol solution to make a concentration of 0~2.4×10 -4 M's solution. Measure the fluorescence emission spectra of different concentrations of iron ions to compound (1), such as image 3 shown. The results showed that with the continuous increase of iron ion concentration in the system, the fluorescence intensity of the compound decreased, which indicated that the compound could be used as a fluorescent probe for sensitive detection of iron ion.
[0040] Compound (2) was prepared as 1×10 -4 M ethanol solution, dissolving iron ions in ethanol solution to make a concentration of 0~2.4×10 -4 M's solution. Measure the fluorescence spectra of different concentrations of iron ions to compound (2), such as Figure 4 shown. The results showed that in the process of increasing the concentration of iron ions, the fluorescence intensity of the compound decreased continuously until it was comple...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com