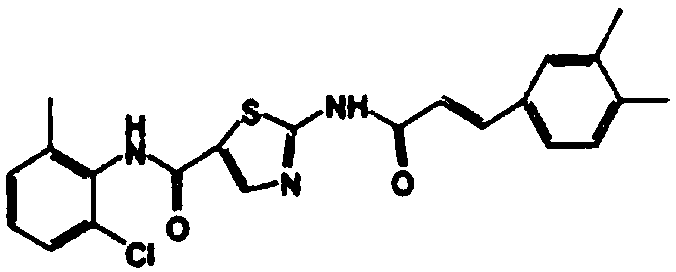
Synthesis process of dasatinib analogue
A synthesis process and dasatinib technology, which are applied in the field of dasatinib analog synthesis technology, can solve the problems of low bioavailability of drug tablets, low yield, and high preparation cost of dasatinib, and achieve The effect of improving bioavailability, high yield, and shortening synthesis time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A synthesis technique of dasatinib analogues, comprising the following steps:
[0029] A. Preparation of Intermediate I
[0030] Add xylene and a catalyst into the reaction kettle, feed nitrogen to exhaust the air, then feed CO, after the reaction is complete, the product is subjected to oil-water separation, washing, drying and distillation to obtain 3-5-dimethylbenzaldehyde; then Add the 3-5-dimethylbenzaldehyde, malonic acid, pyridine, and piperidine into another reactor, stir evenly, and heat until the reaction is complete, then add toluene and potassium carbonate solution in the reactor, Adjust the pH value to 8, continue to stir and react for 35 minutes, separate the liquid, take the water layer, then adjust the pH value of the liquid to 3, filter, take the filter residue, wash with water 3 times, and dry to obtain the intermediate product I;
[0031] B. Preparation of Intermediate II
[0032] Add the intermediate product I prepared in step A into the reaction k...
Embodiment 2
[0043] A synthesis technique of dasatinib analogues, comprising the following steps:
[0044] A. Preparation of Intermediate I
[0045] Add xylene and a catalyst into the reaction kettle, feed nitrogen to exhaust the air, then feed CO, after the reaction is complete, the product is subjected to oil-water separation, washing, drying and distillation to obtain 3-5-dimethylbenzaldehyde; then Add the 3-5-dimethylbenzaldehyde, malonic acid, pyridine, and piperidine into another reactor, stir evenly, and heat until the reaction is complete, then add toluene and potassium carbonate solution in the reactor, Adjust the pH value to 10, continue to stir and react for 45 minutes, separate the liquid, take the water layer, then adjust the pH value of the liquid to 4, filter, take the filter residue, wash with water 3 times, and dry to obtain the intermediate product I;
[0046] B. Preparation of Intermediate II
[0047] Add the intermediate product I prepared in step A into the reaction ...
Embodiment 3
[0058] A synthesis technique of dasatinib analogues, comprising the following steps:
[0059] A. Preparation of Intermediate I
[0060] Add xylene and a catalyst into the reaction kettle, feed nitrogen to exhaust the air, then feed CO, after the reaction is complete, the product is subjected to oil-water separation, washing, drying and distillation to obtain 3-5-dimethylbenzaldehyde; then Add the 3-5-dimethylbenzaldehyde, malonic acid, pyridine, and piperidine into another reactor, stir evenly, and heat until the reaction is complete, then add toluene and potassium carbonate solution in the reactor, Adjust the pH value to 9 and continue to stir for 40 n, separate the liquid, take the water layer, then adjust the pH value of the liquid to 4, filter, take the filter residue, wash with water 3 times, and dry to obtain the intermediate product I;
[0061] B. Preparation of Intermediate II
[0062] Add the intermediate product I prepared in step A into the reaction kettle, then a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com