Method for purification of salt-making brine by recycling sodium carbonate solution
A technology of brine purification and heat recovery, which is applied in the fields of alkali metal halide purification, alkali metal chloride, application, etc., can solve the problems of soda ash consumption and benefit loss, and achieve the effects of reducing environmental pollution, reducing use, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation steps:
[0029] A) The original halogen is introduced into the primary reactor, and the caustic soda solution is added to fully stir the reaction. The content of NaOH in the caustic soda solution is 10mol / L, the volume ratio of the caustic soda solution to the original halogen is 1:100, and the reaction time is 30 minutes. primary slurry;
[0030] B) Settling the primary slurry obtained in step A), adding a flocculant, the addition amount is 1ppm, and the settling time is 60 minutes to obtain the primary supernatant and the primary lower layer mud;
[0031] C) the first-level supernatant liquid obtained in step B) is introduced into the secondary reactor, and the first-level lower layer mud is discharged to the mud collection device, and the hot lye discharged by the hot lye tower is introduced into the secondary reactor simultaneously, The temperature of the introduced hot lye is 70°C, Na in the hot lye 2 CO 3 The content of lye is 10mol / L, the volume ra...
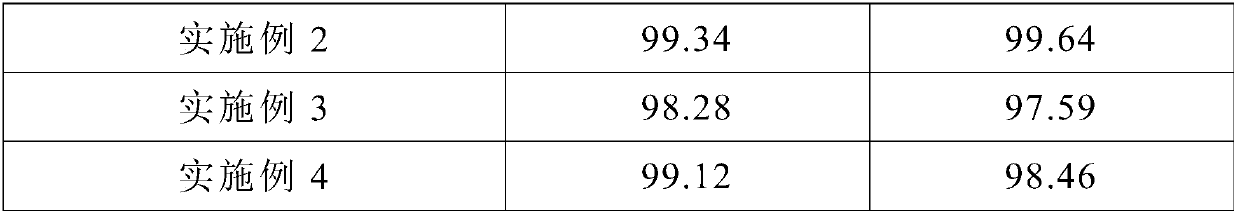
Embodiment 2
[0035] Preparation steps:
[0036] A) The original halogen is introduced into the primary reactor, and the caustic soda solution is added to fully stir the reaction. The content of NaOH in the caustic soda solution is 19mol / L, the volume ratio of the caustic soda solution to the original halogen is 3:100, and the reaction time is 100 minutes. primary slurry;
[0037] B) Settling the primary slurry obtained in step A), adding a flocculant, the addition amount is 5ppm, and the settling time is 150 minutes to obtain the primary supernatant and the primary mud;
[0038] C) the first-level supernatant liquid obtained in step B) is introduced into the secondary reactor, and the first-level lower layer mud is discharged to the mud collection device, and the hot lye discharged by the hot lye tower is introduced into the secondary reactor simultaneously, The temperature of the introduced hot lye is 80°C, Na in the hot lye 2 CO 3 The content of lye is 2.5mol / L, the volume ratio of th...
Embodiment 3
[0042] Preparation steps:
[0043] A) The original halogen is introduced into the primary reactor, and the caustic soda solution is added to fully stir the reaction. The content of NaOH in the caustic soda solution is 15mol / L, the volume ratio of the caustic soda solution to the original halogen is 2:100, and the reaction time is 60 minutes. primary slurry;
[0044] B) Settling the primary slurry obtained in step A), adding a flocculant, the addition amount is 2ppm, and the settling time is 100 minutes to obtain the primary supernatant and the primary mud;
[0045] C) the first-level supernatant liquid obtained in step B) is introduced into the secondary reactor, and the first-level lower layer mud is discharged to the mud collection device, and the hot lye discharged by the hot lye tower is introduced into the secondary reactor simultaneously, The temperature of the introduced hot lye is 75°C, Na in the hot lye 2 CO 3The content of lye is 1.5mol / L, the volume ratio of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com