A kind of method for preparing diethoxymethane
A diethoxymethane and formaldehyde technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as long contact time, affecting product quality and yield, affecting conversion rate, etc., to reduce The production of monoethoxymethane, the effect of avoiding coking and deactivation, and avoiding secondary reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
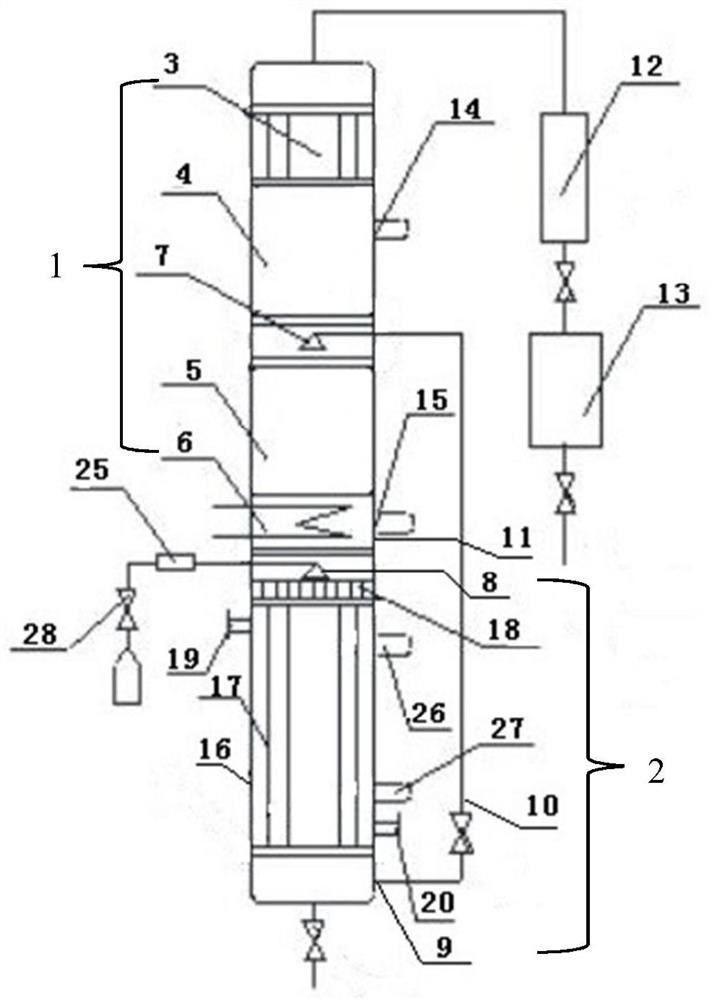
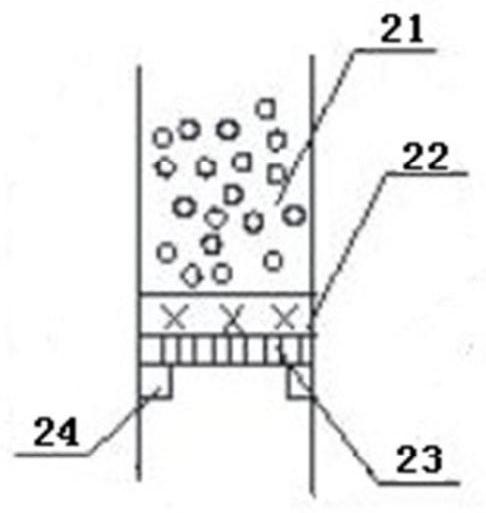
[0019] The upper end of the tube bundle reaction device 2 is connected to the rectifying device 1 by a flange, and the temperature of the reaction tube 12 in the tube bundle reaction device 2 is controlled by adding a heating medium from the heating medium feed port 15 to be 101 to 103° C. and the temperature of the preheater 25 The temperature is 102-104°C, while the temperatures of the inner reflux condenser 3 and the outer condenser 16 are controlled by cooling medium, and the temperatures are 65.0-68.0°C and 22.0-28.0°C respectively. The packing in the packing 2 of the rectification section adopts Ring, the packing in the packing 4 of the stripping section adopts ring.
[0020] Mix ethanol and formaldehyde (its mass percentage is 37%) according to the mass ratio of 1.2:1, and control the air speed ratio by valve 26 to be 1.0h -1 , after the mixture is preheated, it is fed into the reaction tube 12 from the second feed port 8 through the distributor 13, and SO is adde...
Embodiment 2
[0022] The basic steps are the same as in Example 1, except that the mass ratio of ethanol and formaldehyde is 1.15:1, SO 4 2- The mass ratio of / Hβ catalyst to formaldehyde is 4.5:100, reacts under the action of catalyst, generates the mixture containing 57.13% diethoxymethane quality, the reflux ratio of the internal reflux condenser 3 of setting is 2, low boiling point The components are refluxed through the condensed part of the internal reflux condenser 3, and the remaining part is condensed through the external condenser 16 to obtain a mixture with a mass content of 91.94%. The yield of diethoxymethane reaches 99.02%, and the material is discharged to the collection tank 17, while Water content of 98.21% and unreacted ethanol flow out from the first outlet 20 at the bottom of the rectification device 1 .
Embodiment 3
[0024] The basic steps are the same as in Example 1, except that the mass ratio of ethanol and formaldehyde is 1.25:1, SO 4 2- The mass ratio of / Hβ catalyst to formaldehyde is 6.5:100, reacts under the action of catalyst, generates the mixture that contains 58.31% diethoxymethane quality, the reflux ratio of setting internal reflux condenser 3 is 4, low boiling point The components are refluxed through the condensed part of the internal reflux condenser 3, and the remaining part is condensed through the external condenser 16 to obtain a mixture with a mass content of 92.22%. The yield of diethoxymethane reaches 99.47%, and the material is discharged to the collection tank 17, and Water content of 98.37% and unreacted ethanol flow out from the first outlet 20 at the bottom of the rectification device 1 .
[0025] By adopting the preparation method of the present invention to prepare diethoxymethane, after 700 hours of work, the purity of diethoxymethane only decreases by 0.15...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com