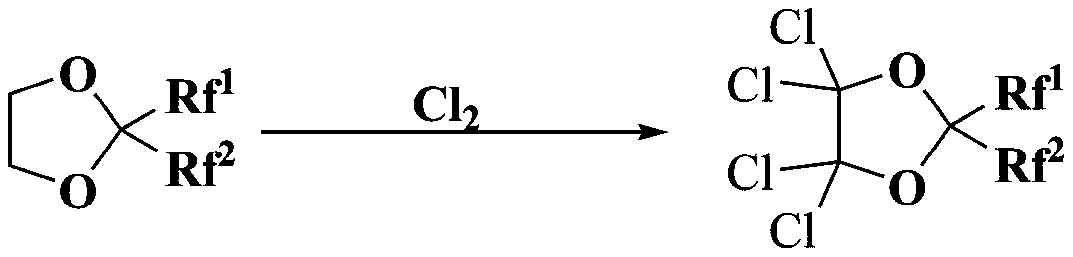A kind of synthetic method of chloro-2,2-bis(perfluoro substituent)-1,3-dioxolane compounds
A technology of dioxolane and fluorine substituents, which is applied in the field of synthesis of fluorine-containing organic compounds, and can solve the problems of affecting product quality stability, large axial temperature difference of reactor, and easy deactivation of catalysts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] In the autoclave, 0.2 moles of 2,2-bis(trifluoromethyl)-1,3-dioxolane, 0.02 moles of CuCl 2 2H 2 O, replace the air in the reactor with nitrogen at a pressure of 0.5 MPa three times, pass 0.8 moles of chlorine gas under stirring, the reaction starting temperature is 25-30 ° C, gradually increase the temperature to 150 ° C, keep 150 ° C for 3 hours and stop heating And stir, cool to room temperature, and slowly vent the pressure to normal pressure. The reaction solution was taken out, washed with water, washed with alkali, dried, and analyzed by a hydrogen flame ionization detector (FID), including 0.7% of 2,2-bis(trifluoromethyl)-1,3-dioxolane, 4,5-dichloro-2,2-bis(trifluoromethyl)-1,3-dioxolane 3%, 4,4-dichloro-2,2-bis(trifluoromethyl) -1,3-dioxolane 1%, 4,4,5-trichloro-2,2-bis(trifluoromethyl)-1,3-dioxolane 2%, 4, 4,5,5-tetrachloro-2,2-bis(trifluoromethyl)-1,3-dioxolane 91.2%, unknown component 2.1%.
Embodiment 2
[0032] In the autoclave, 0.2 moles of 2,2-bis(trifluoromethyl)-1,3-dioxolane, 0.02 moles of CuCl 2 2H 2O, replace the air in the reactor with nitrogen at a pressure of 0.5 MPa three times, pass 0.8 moles of chlorine gas under stirring, the initial reaction temperature is 25-30°C, gradually increase the temperature to 200°C, keep the reaction at 200°C for 3 hours, then stop heating And stir, cool to room temperature, and slowly vent the pressure to normal pressure. The reaction solution was taken out, washed with water, washed with alkali, dried, and analyzed by a hydrogen flame ionization detector (FID), including 0.2% of 2,2-bis(trifluoromethyl)-1,3-dioxolane, 4,4,5-Trichloro-2,2-bis(trifluoromethyl)-1,3-dioxapentane 1.2%, 4,4,5,5-tetrachloro-2,2-bis(tri Fluoromethyl)-1,3-dioxolane 96.0%, carbon tetrachloride 1.2%, unknown components 1.4%.
Embodiment 3
[0034] In the autoclave, 0.2 moles of 2,2-bis(trifluoromethyl)-1,3-dioxolane, 0.02 moles of CuCl 2 2H 2 O, replace the air in the reactor with nitrogen at a pressure of 0.5MPa three times, pass 1.2 moles of chlorine gas under stirring, the initial reaction temperature is 25-30°C, gradually increase the temperature to 150°C, keep the reaction at 150°C for 3 hours, then stop heating And stir, cool to room temperature, and slowly vent the pressure to normal pressure. The reaction solution was taken out, washed with water, washed with alkali, dried, and analyzed by a hydrogen flame ionization detector (FID), including 0.1% of 2,2-bis(trifluoromethyl)-1,3-dioxolane, 4,4,5-Trichloro-2,2-bis(trifluoromethyl)-1,3-dioxapentane 0.8%, 4,4,5,5-tetrachloro-2,2-bis(tri Fluoromethyl)-1,3-dioxolane 97%, unknown components 2.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com